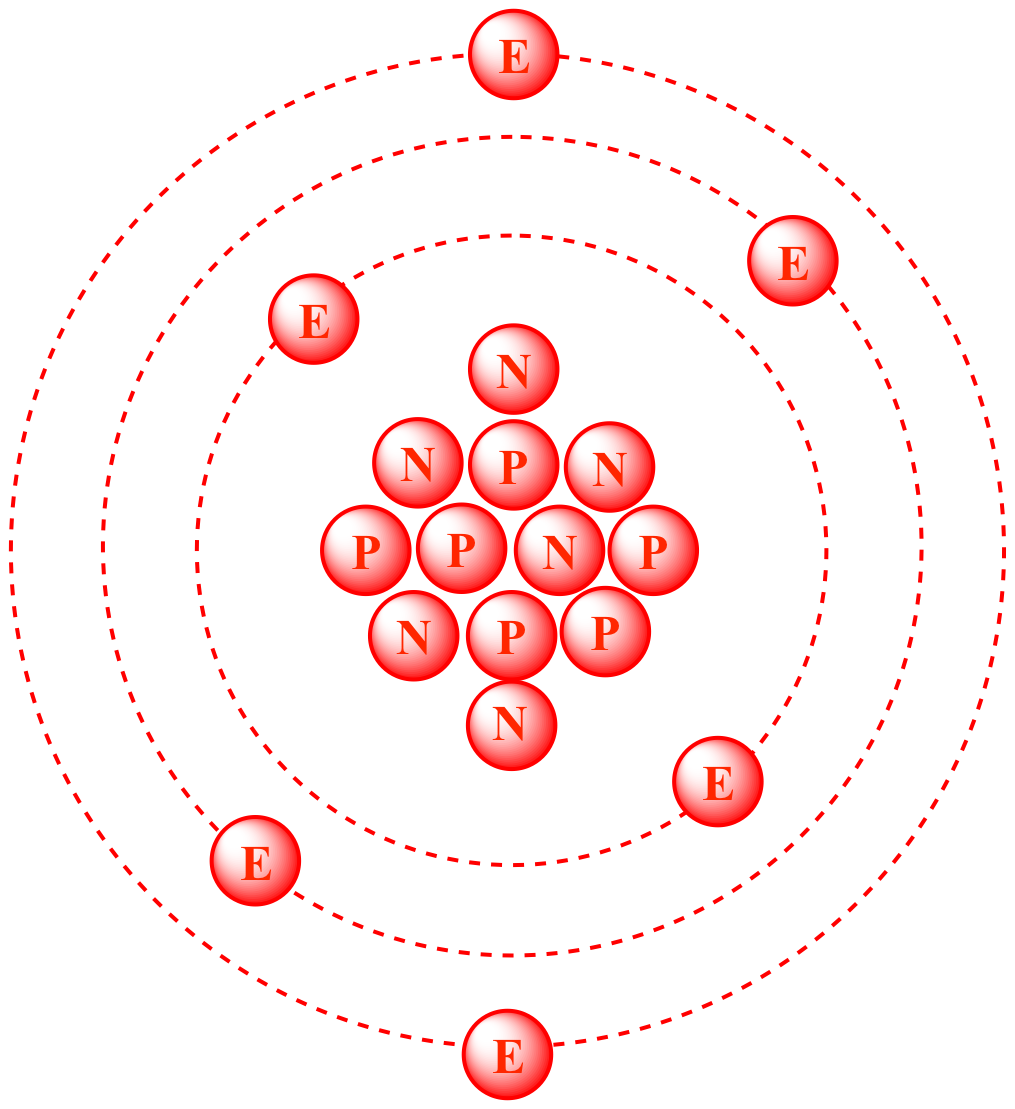
Calculating Average Atomic Mass The atomic mass of an element is a weighted average of the different isotopes of a naturally occurring sample of the element. - ppt download

Draw the circular diagram to represent the atomic mass of carbon-12for one atomic mass unit - Brainly.in

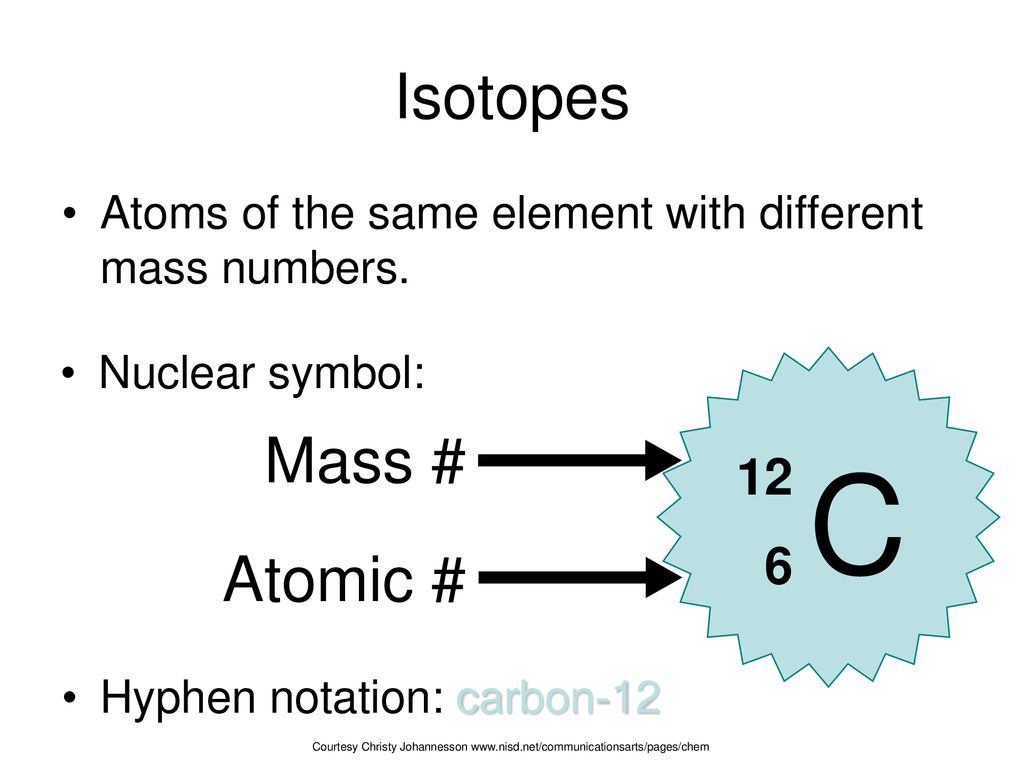
1.4 Isotopes, Radioisotopes, and Atomic Mass B3.1 explain the relationship between the atomic number and the mass number of an element, and the difference. - ppt download
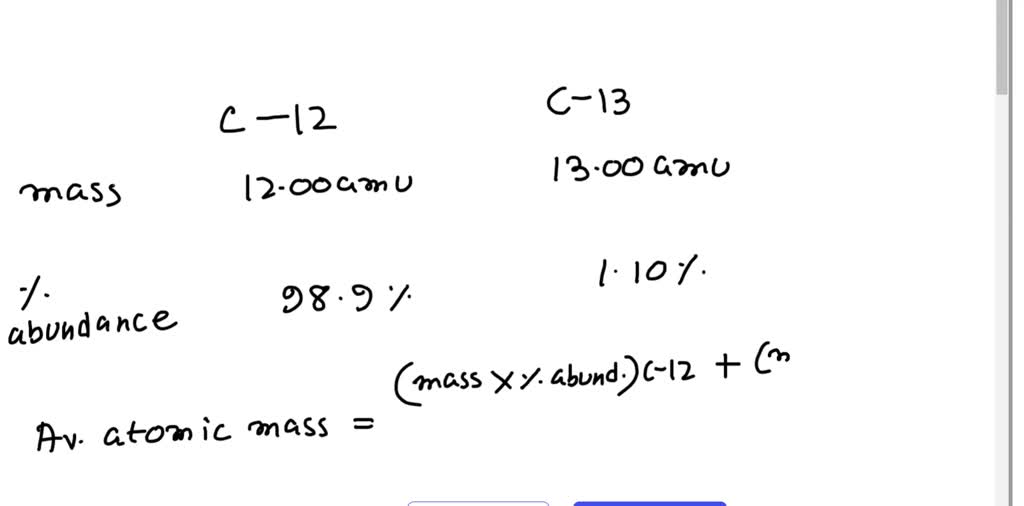
SOLVED: The two most abundant isotopes of carbon are carbon-12 (mass = 12.00 amu) and carbon-13 (mass = 13.00 amu). Their relative abundances are 98.9% and 1.10%, respectively. Calculate the average atomic mass of carbon.

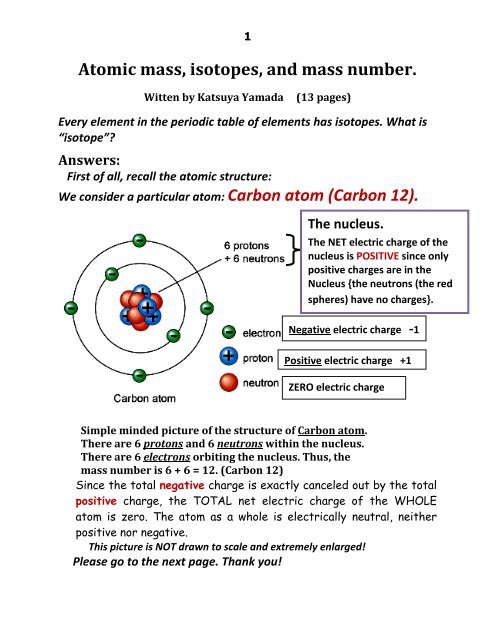
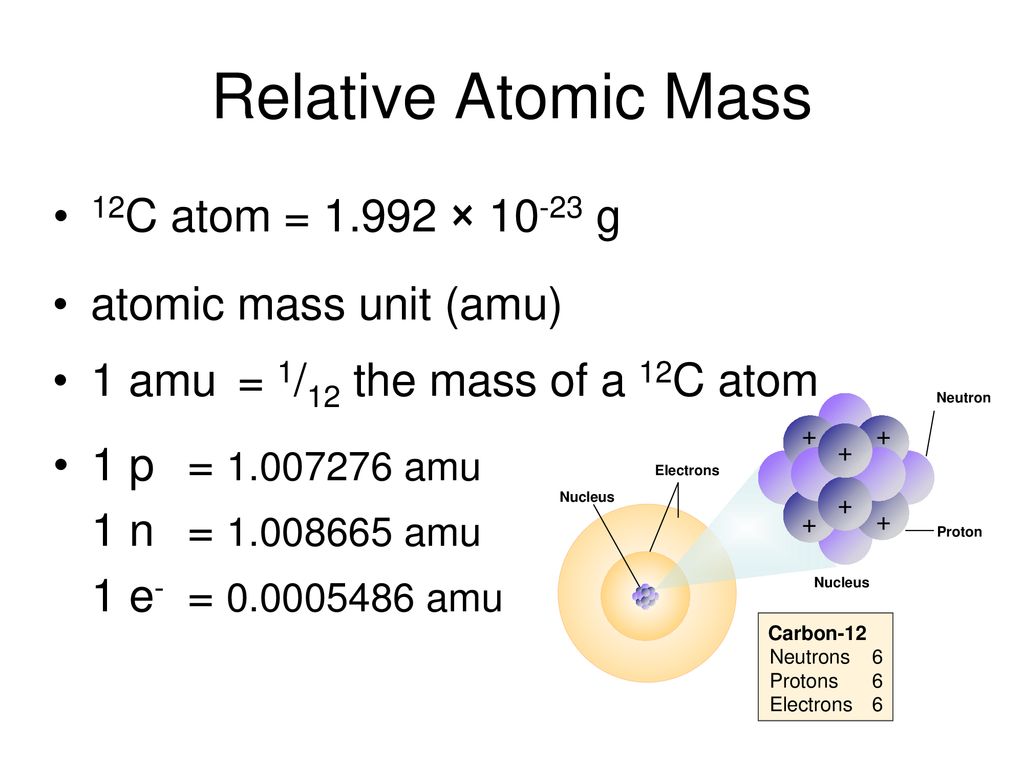
Atomic Mass Standard mass unit is derived from carbon 12 Atomic mass unit – the mass equal to 1/12 the mass of one Carbon 12 atom. - ppt download
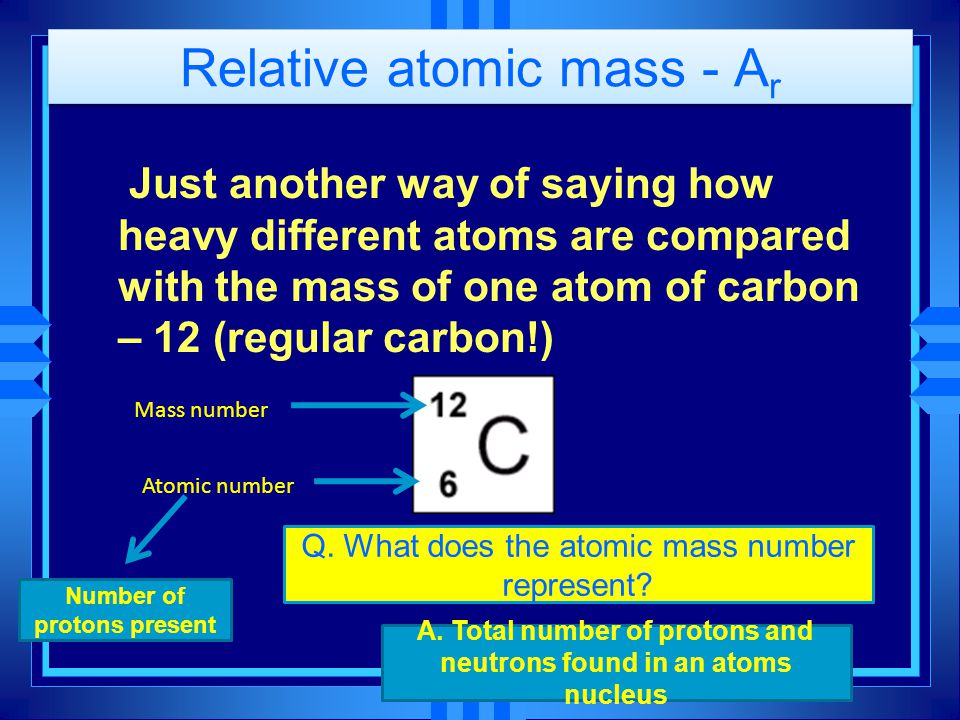
Relative atomic mass - A r Just another way of saying how heavy different atoms are compared with the mass of one atom of carbon – 12 (regular carbon!) - ppt download










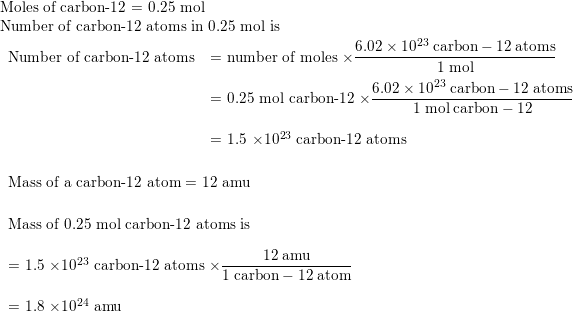
:max_bytes(150000):strip_icc()/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)