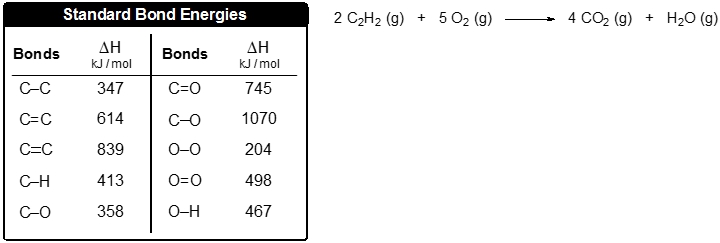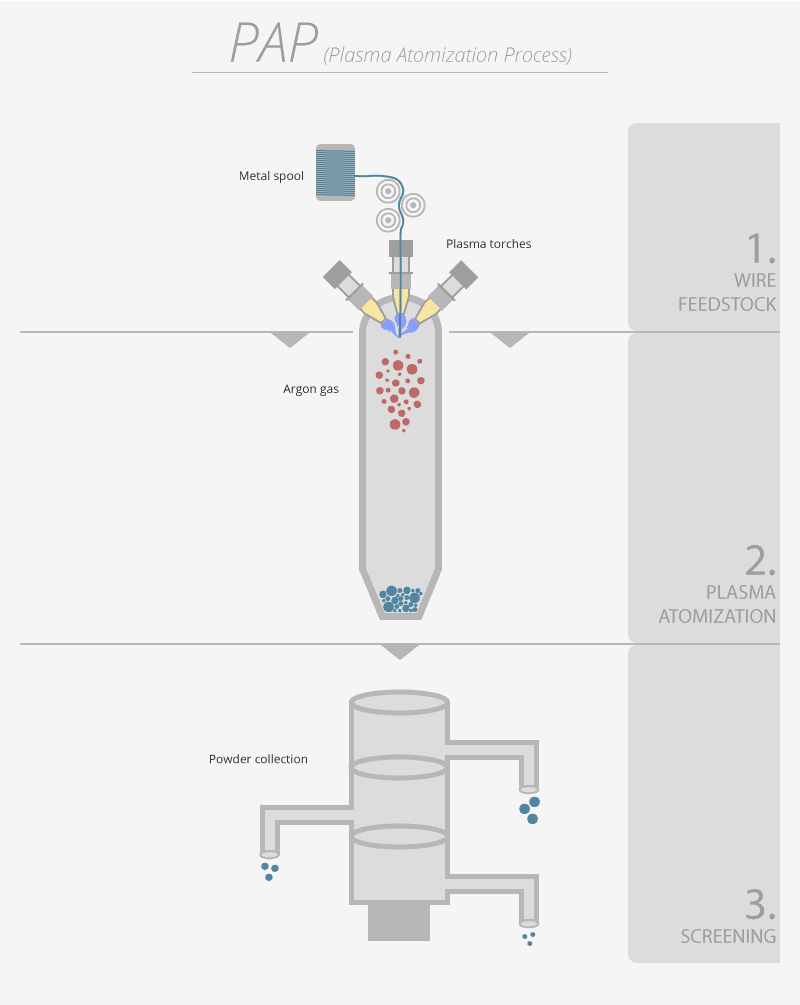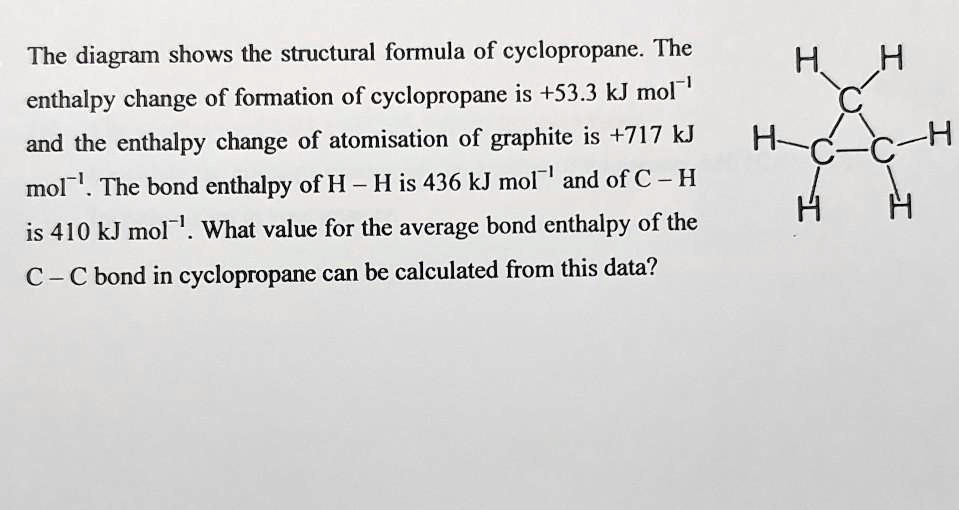
SOLVED: The diagram shows the structural formula of cyclopropane. The enthalpy change of formation of cyclopropane is +53.3 kJ mol-! and the enthalpy change of atomisation of graphite is +717 kJ mol-I

Investigations on the determination of germanium in organogermanium compounds using carbon furnace atomisation - Analyst (RSC Publishing)
Selective state-space enrolment in energy-carbon restructuring: syndromic experimentation and atomisation in England

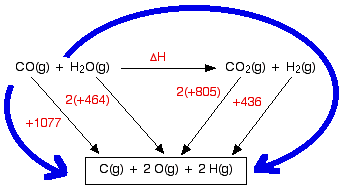
Enthalpy of atomisation of C = a Bond enthalpy of H2 = b Enthalpy of formation of CH4 = c Enthalpy of formation of C2H6 = d What is the C - C bond energy?

SOLVED: The diagram shows the structural formula of cyclopropane. The enthalpy change of formation of cyclopropane is +53.3 kJ mol-! and the enthalpy change of atomisation of graphite is +717 kJ mol-I
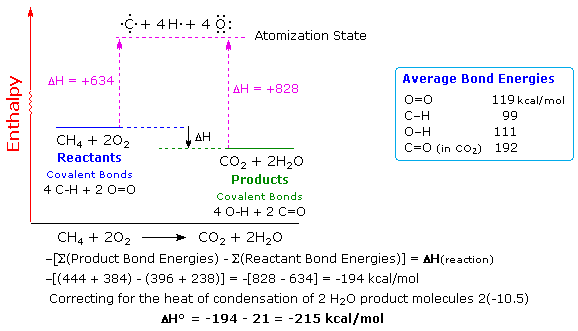
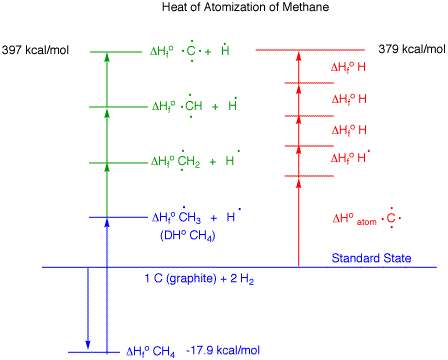
What is the difference between bond dissociation enthalpy and enthalapy of atomization? I'm reading it through NCERT and same example of H2(g) ____ 2H(g) and CH4(g) ____C(g) +4H(g) is given in both. -

If EC - C is 344 kJ mole^-1 and EC - H is 415 kJ mole^-1 , calculate the heat of formation of propane. The heats of atomization of carbon and hydrogen
![Erratum: “An accurate and transferable machine learning potential for carbon” [J. Chem. Phys. 153, 034702 (2020)]: The Journal of Chemical Physics: Vol 156, No 15 Erratum: “An accurate and transferable machine learning potential for carbon” [J. Chem. Phys. 153, 034702 (2020)]: The Journal of Chemical Physics: Vol 156, No 15](https://aip.scitation.org/action/showOpenGraphArticleImage?doi=10.1063/5.0091698&id=images/medium/5.0091698.figures.online.f1.jpg)
Erratum: “An accurate and transferable machine learning potential for carbon” [J. Chem. Phys. 153, 034702 (2020)]: The Journal of Chemical Physics: Vol 156, No 15
heat of formation of ethane 20.2kcal /mol and heat of atomisation of C and H2 are 179.2kcal /mol and 52.1kcal /mol if bond energy of c h b ond is 73.3 kcal/mol

Applied Sciences | Free Full-Text | Numerical Investigation on a Liquid–Gas Ejector for Carbon Dioxide Removal Using Amine Solution: Hydrodynamics and Mass Transfer Evaluation

Difference Between Enthalpy of Atomisation and Bond Dissociation | Compare the Difference Between Similar Terms

The enthalpy of atomization of graphite is 698.6 kl/mol and the mean bond enthalpy of C - C bond in diamond is 348.4 kJ/mol . The enthalpy of conversion of graphite into