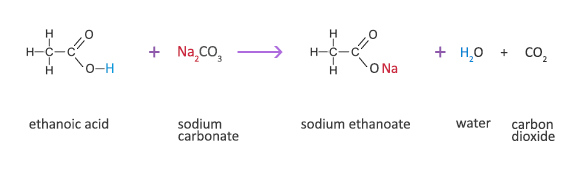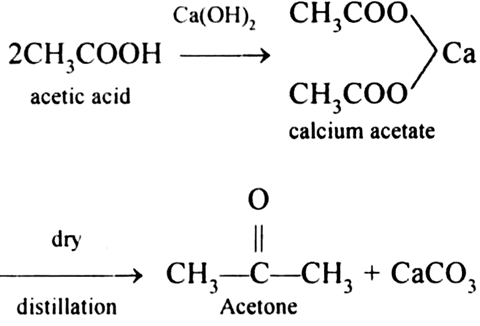
Write reactions for obtaining:Benzene from toluene. from Chemistry Aldehydes, Ketones and Carboxylic Acids Class 12 CBSE

SOLVED:Vinegar, which is an aqueous solution of acetic acid, is used to remove deposits of calcium carbonate (lime scale) from automatic coffee makers. Write the equation describing the reaction between acetic acid

SOLVED: (0.5 mark) Classify the type of reaction that occurs between calcium carbonate and acetic acid. 42 (cH;coo)zCa 4,Oe” (01s CaCO;, 2CH;kcH (4p) Nevtra 14 Le imiine Rrac on mark ) Based

Chemical reactions of two calcium salts (citrate and carbonate) with... | Download Scientific Diagram
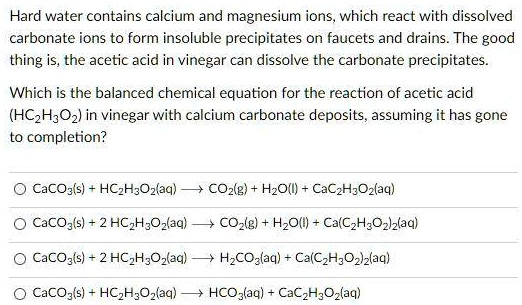
SOLVED: Hard water contains calcium and magnesium ions, which react with dissolved carbonate ions to form insoluble precipitates on faucets and drains. The good thing is, the acetic acid in vinegar can
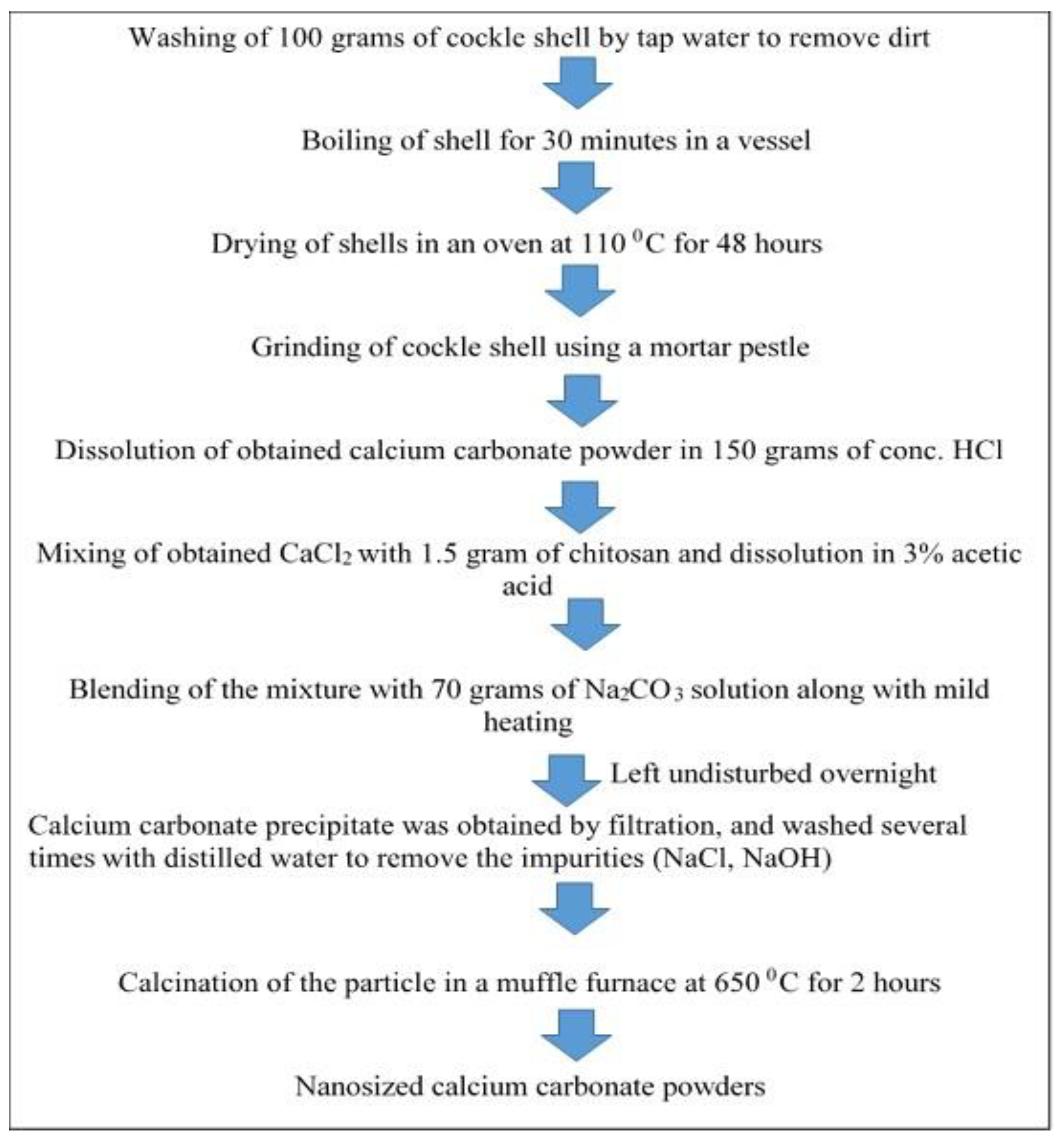
Applied Sciences | Free Full-Text | The Processing of Calcium Rich Agricultural and Industrial Waste for Recovery of Calcium Carbonate and Calcium Oxide and Their Application for Environmental Cleanup: A Review
Explain why does the lime water turns milky in the reaction of acetic acid with sodium carbonate. - Sarthaks eConnect | Largest Online Education Community
What is the Ionic equation for the reaction between calcium carbonate and hydrochloric acid? - Quora
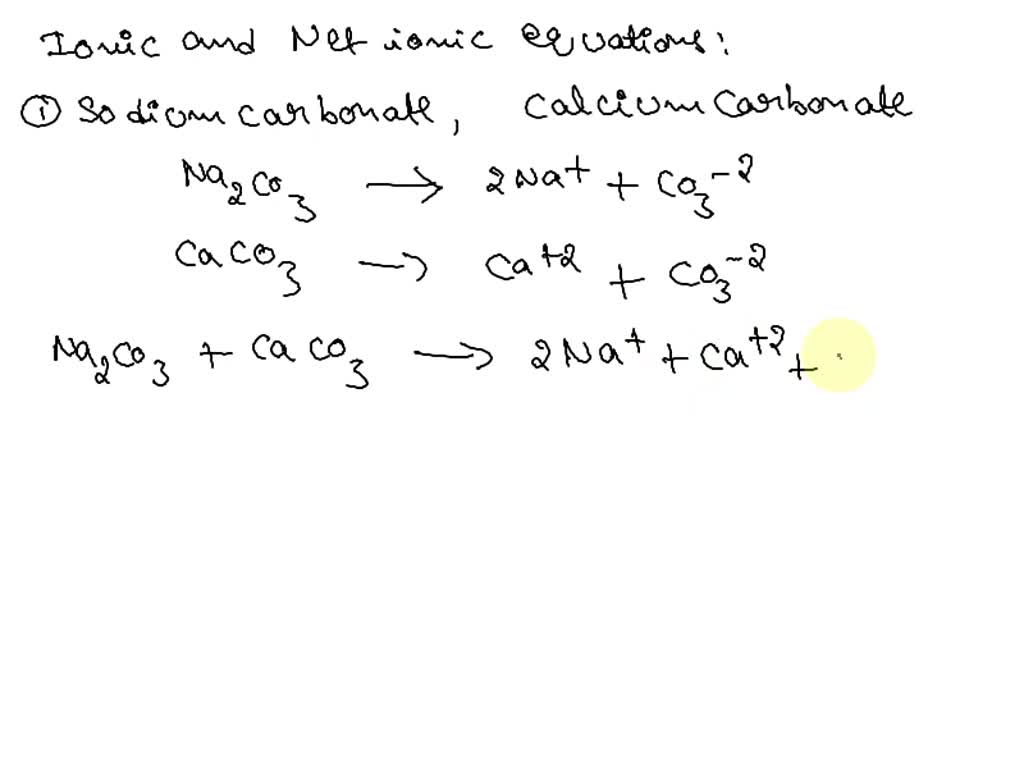
SOLVED: Write a full ionic equation and net ionic equation for sodium carbonate + calcium carbonate, and then for sodium phosphate and calcium carbonate

Heterogeneous reaction of SO2 on CaCO3 particles: Different impacts of NO2 and acetic acid on the sulfite and sulfate formation - ScienceDirect







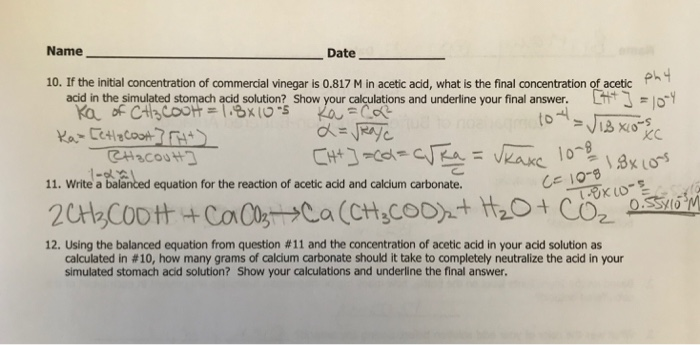



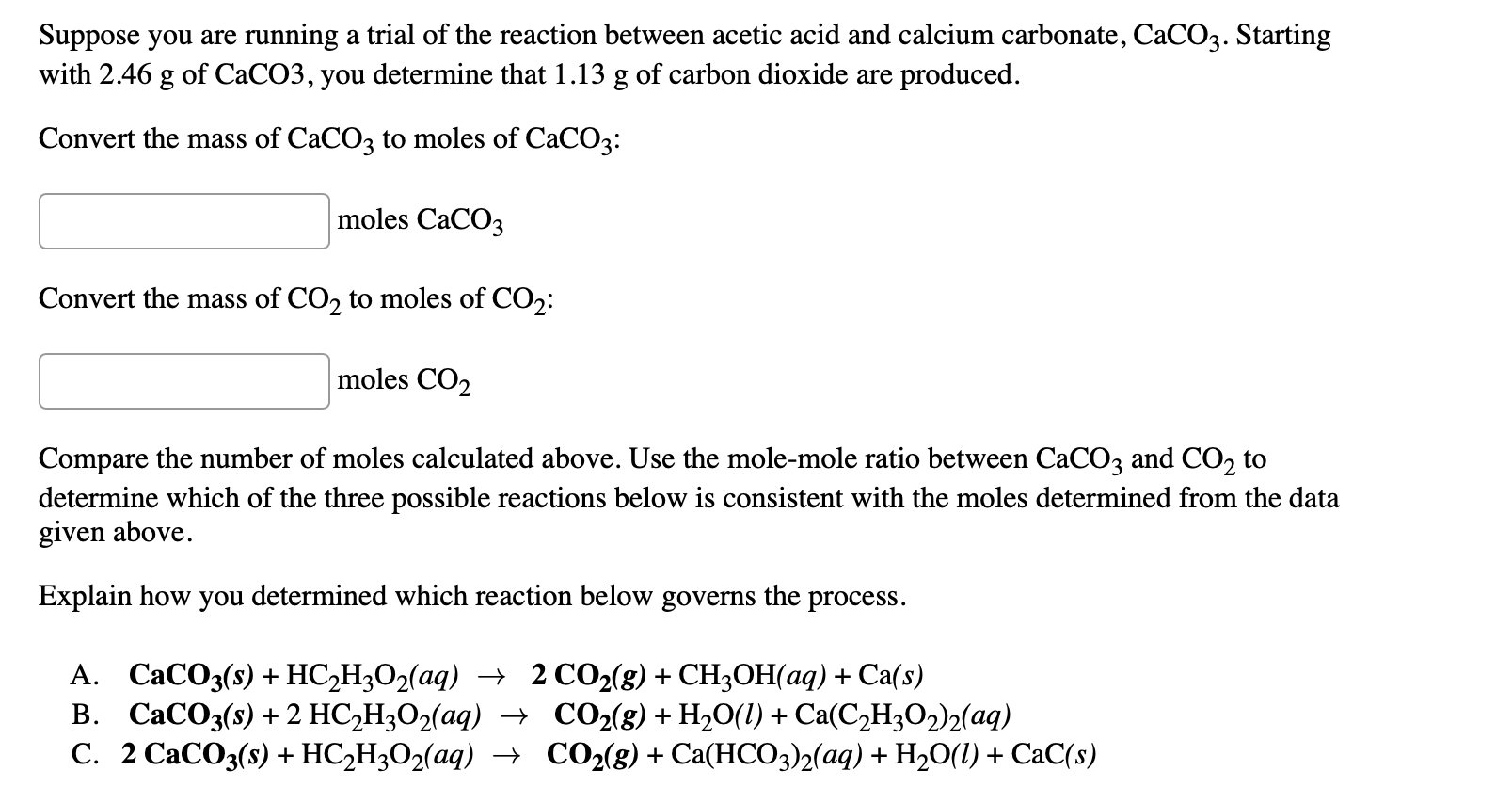
.jpg)
.jpg)