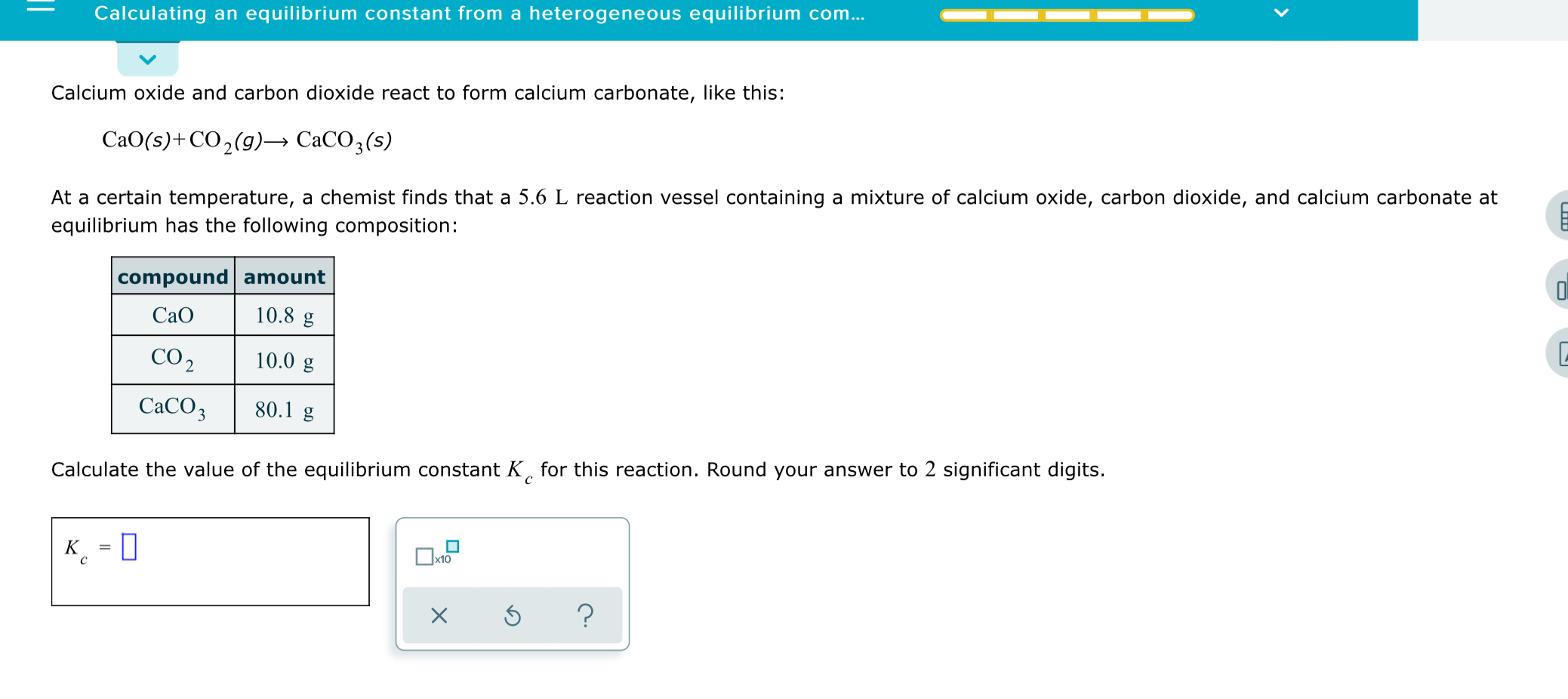
If 220 grams of calcium oxide (CaO) reacts with 50L of carbon dioxide (CO2), what mass of calcium carbonate (CaCO3) is produced? - Quora
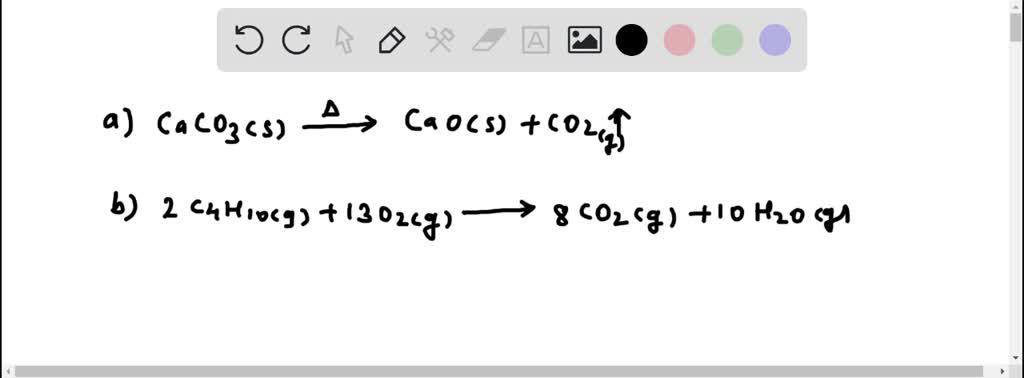
SOLVED:Write a balanced molecular equation describing each of the following chemical reactions. (a) Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. (b) Gaseous butane, C4
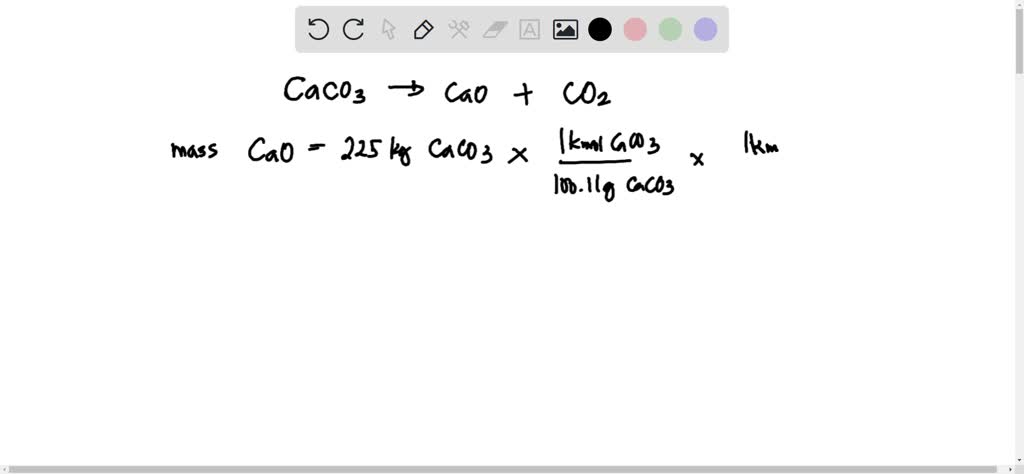
SOLVED: 'Calcium carbonate is heated. Calcium oxide and carbon dioxide gas are formed: The equation for the reaction is shown: CaCO3 Cao COz 225kg of calcium carbonate is heated until there is

Write the balanced chemical equations for the folowing reactions (a) Calcium hydroxide + Carbon - YouTube

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride
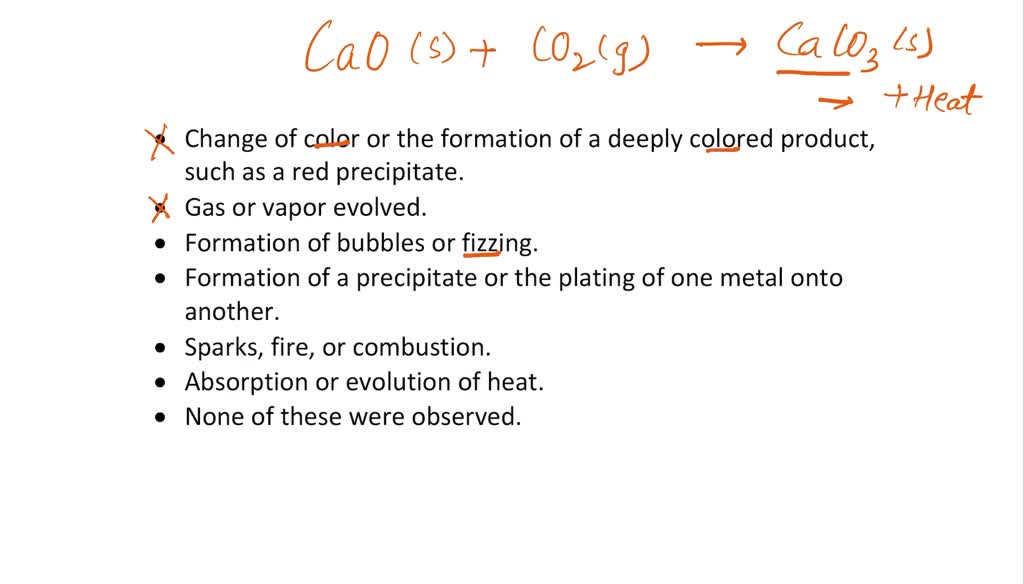
SOLVED: Reaction of calcium oxide and carbon dioxide: Watch the video for this experiment and list each of the changes you observed. Change of color or the formation of deeply colored product