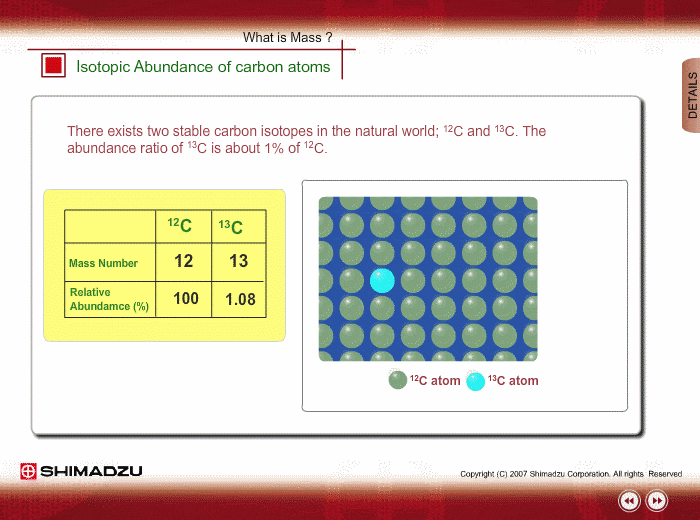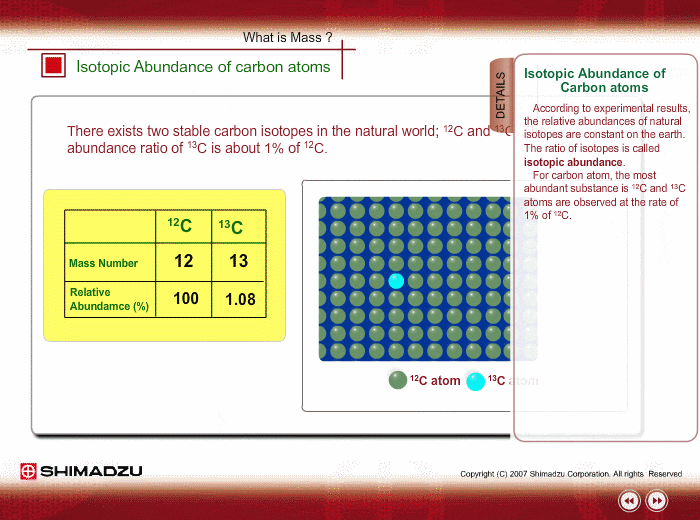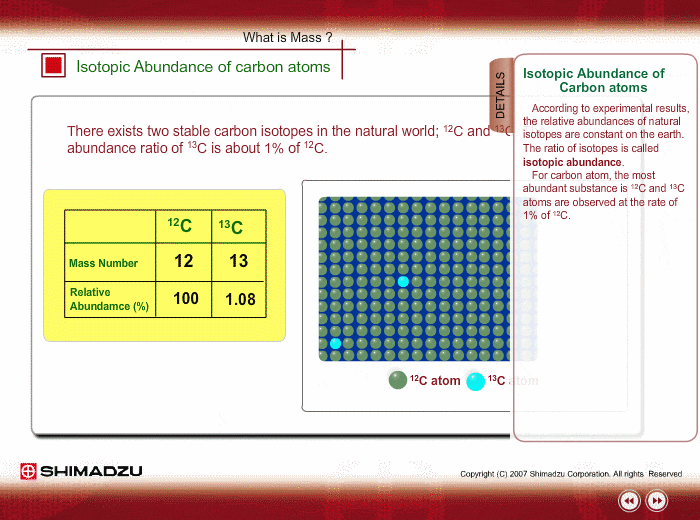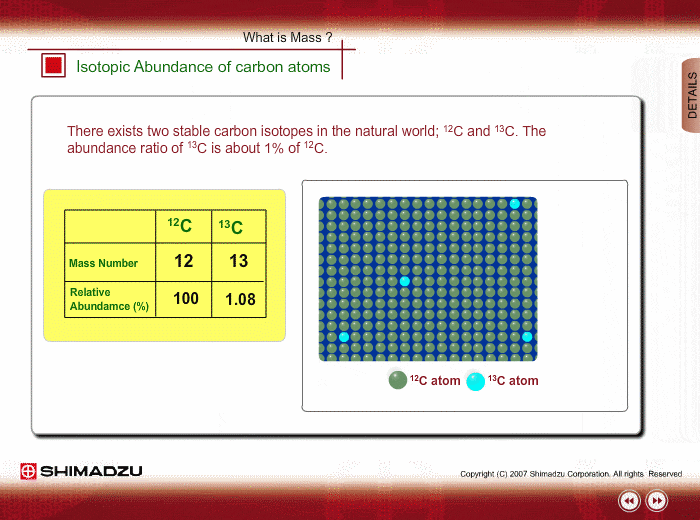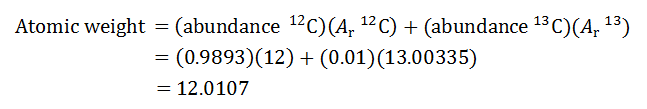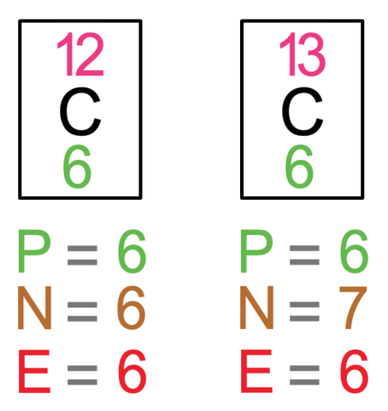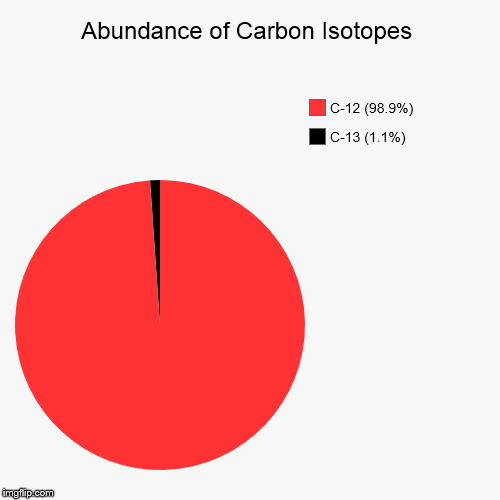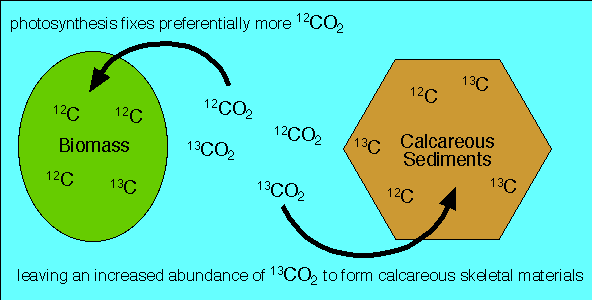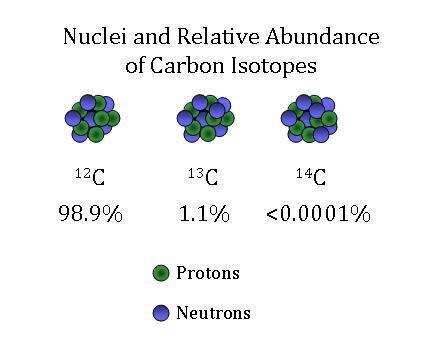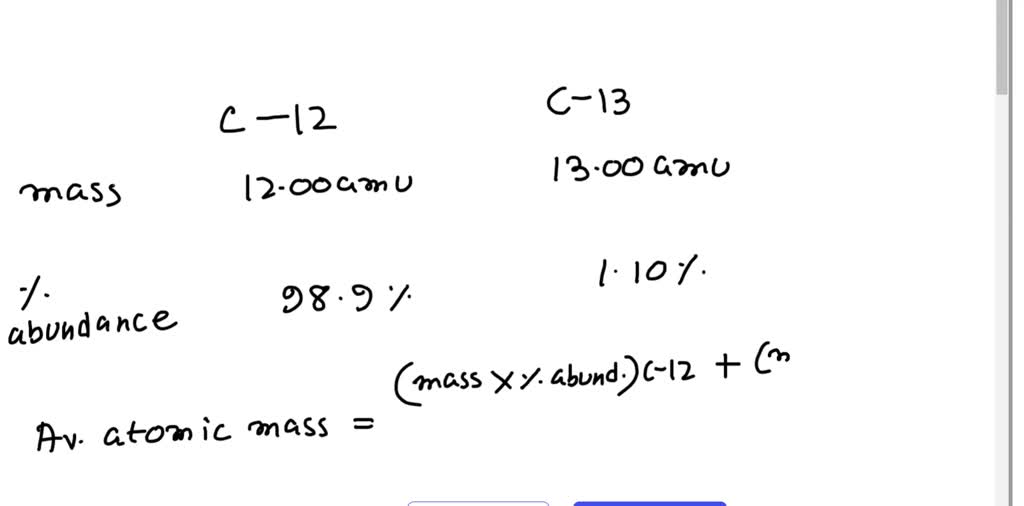
SOLVED: The two most abundant isotopes of carbon are carbon-12 (mass = 12.00 amu) and carbon-13 (mass = 13.00 amu). Their relative abundances are 98.9% and 1.10%, respectively. Calculate the average atomic mass of carbon.

Isotopic Abundance SCH 3U. Atomic Mass The mass of an atom (protons, neutrons, electrons) Relative Atomic Mass: An element's atomic mass relative to the. - ppt download