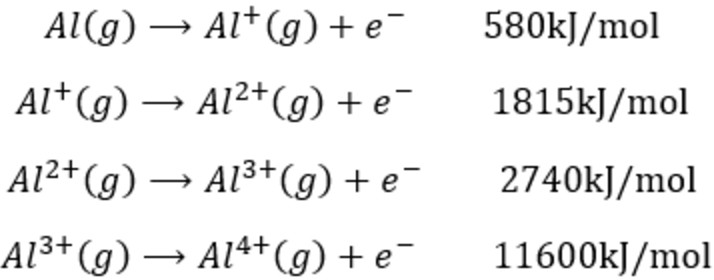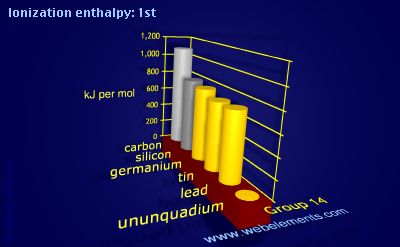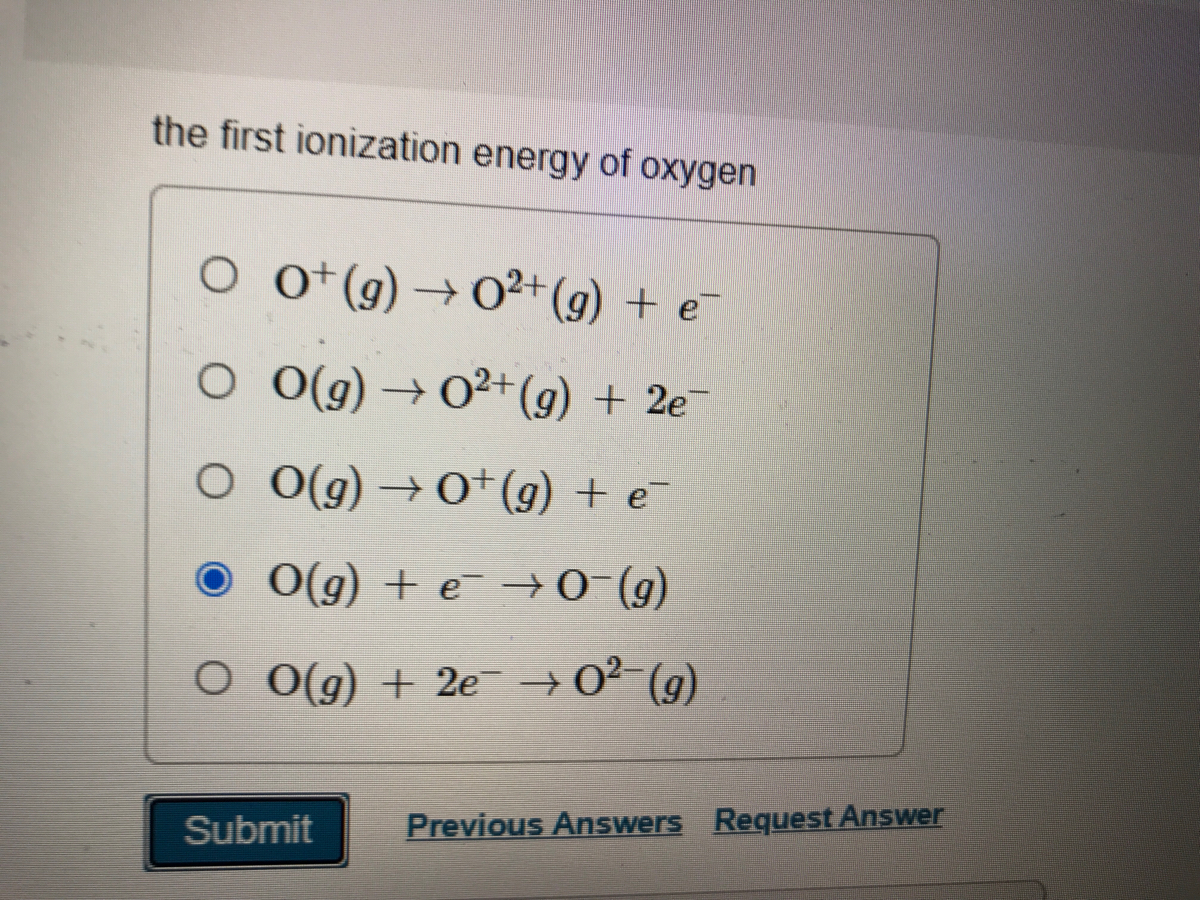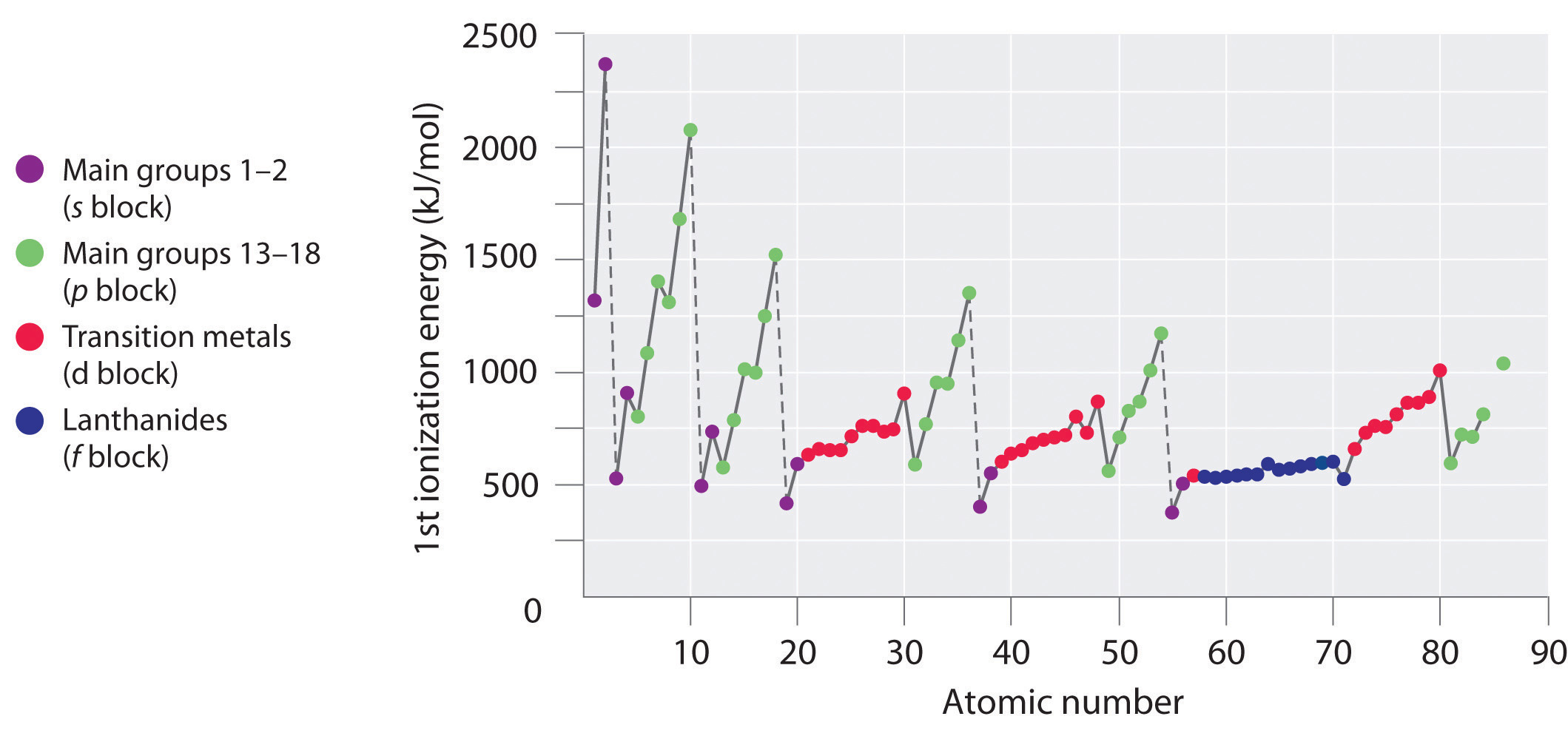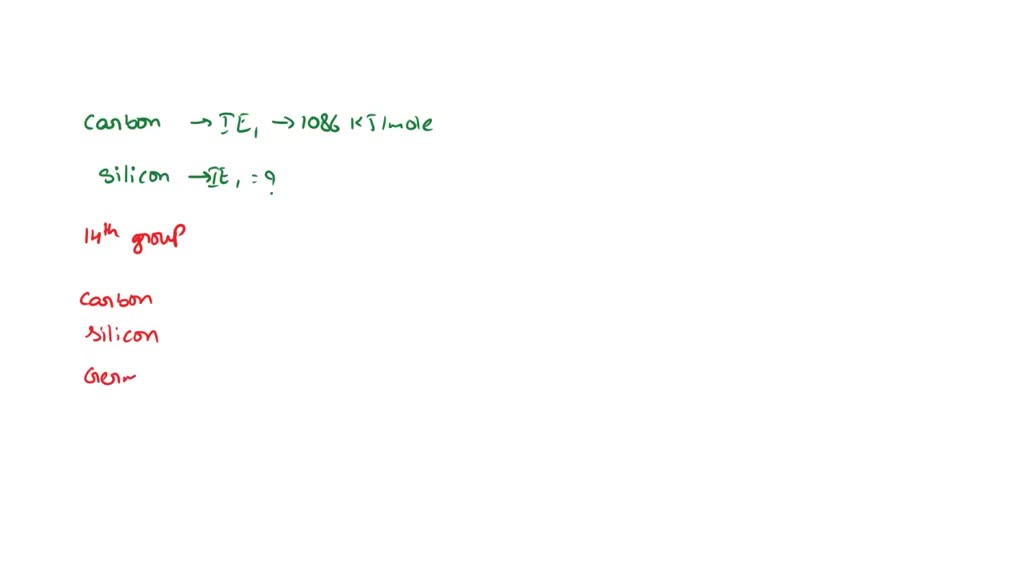
SOLVED: the first ionization energy for carbon is 1086 kJ/mol, then the first ionization energy for silicon will be a) 1086 kJ/mol. c) greater than 1086 kJ/mol. b) close to zero. d)

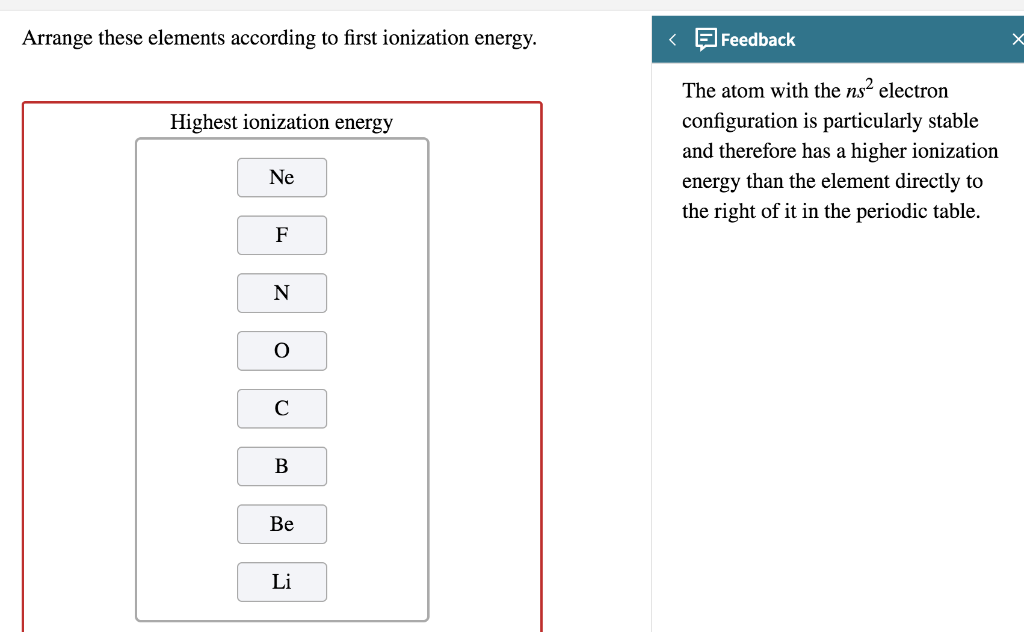
SOLVED: Of the following, which element has the highest first ionization energy? Select one: a. beryllium b. carbon potassium d, boron lithium

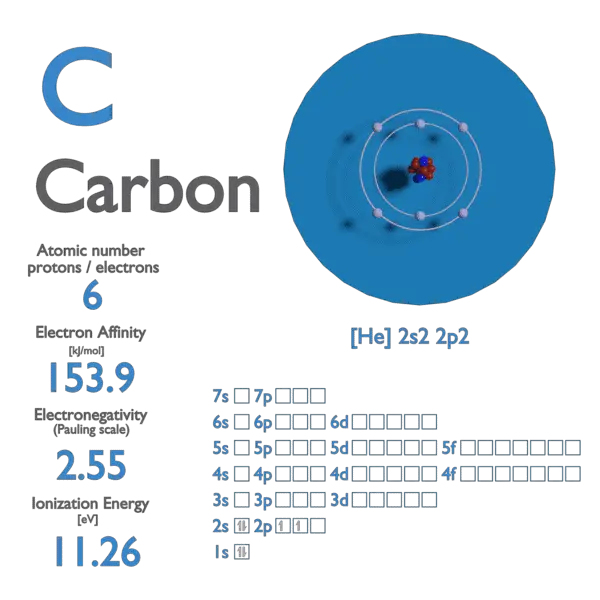
Carbon Chemical Element First Ionization Energy Stock Vector (Royalty Free) 1193986345 | Shutterstock

The first ionization energy of carbon is more than boron, but the second ionization energy is in reverse. Why? - Quora

Premium Vector | Carbon chemical element with first ionization energy atomic mass and electronegativity values on scientific background

Carbon Chemical Element First Ionization Energy Stock Vector (Royalty Free) 2078819101 | Shutterstock