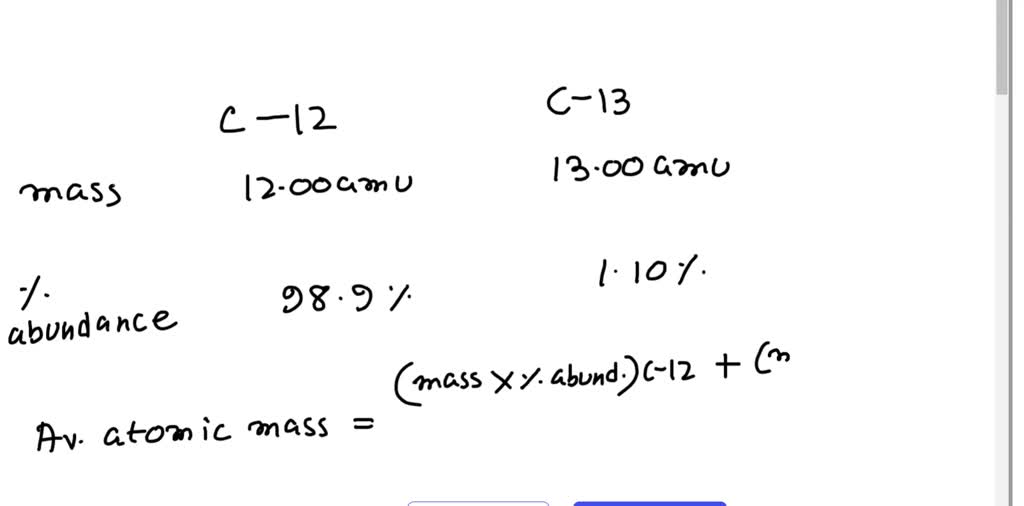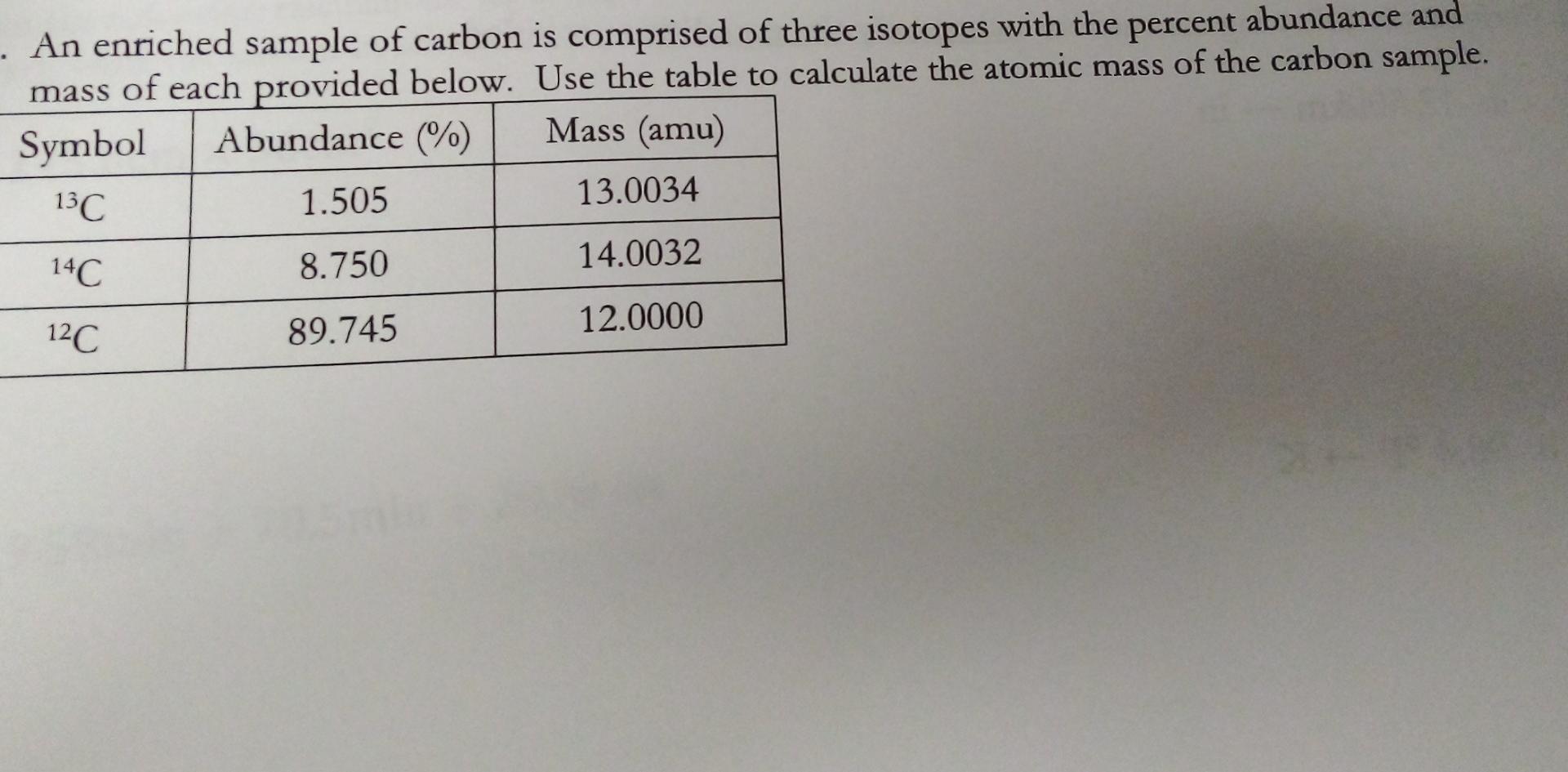The relative abundances of the stable isotopes of elements commonly... | Download Scientific Diagram
How would you calculate the relative abundance for two isotopes when the relative atomic mass is given? - Quora

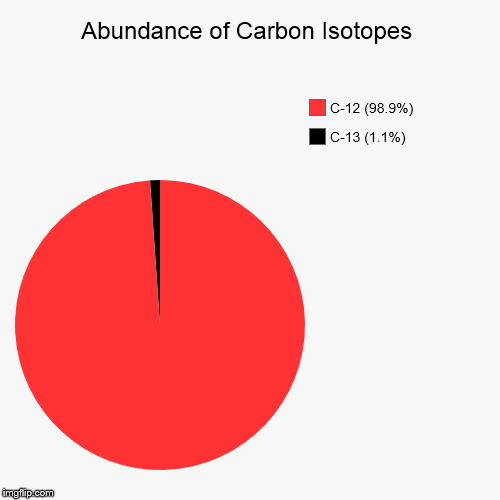
Natural abundances of ^12C and ^13C isotopes of carbon are 99% and 1% , respectively. Assuming they only contribute to the mol. wt. of C2F4 , the percentage of C2F4 having a

Isotopic Abundance SCH 3U. Atomic Mass The mass of an atom (protons, neutrons, electrons) Relative Atomic Mass: An element's atomic mass relative to the. - ppt download