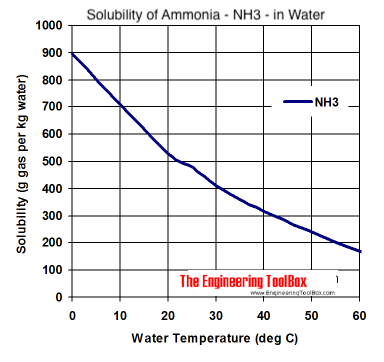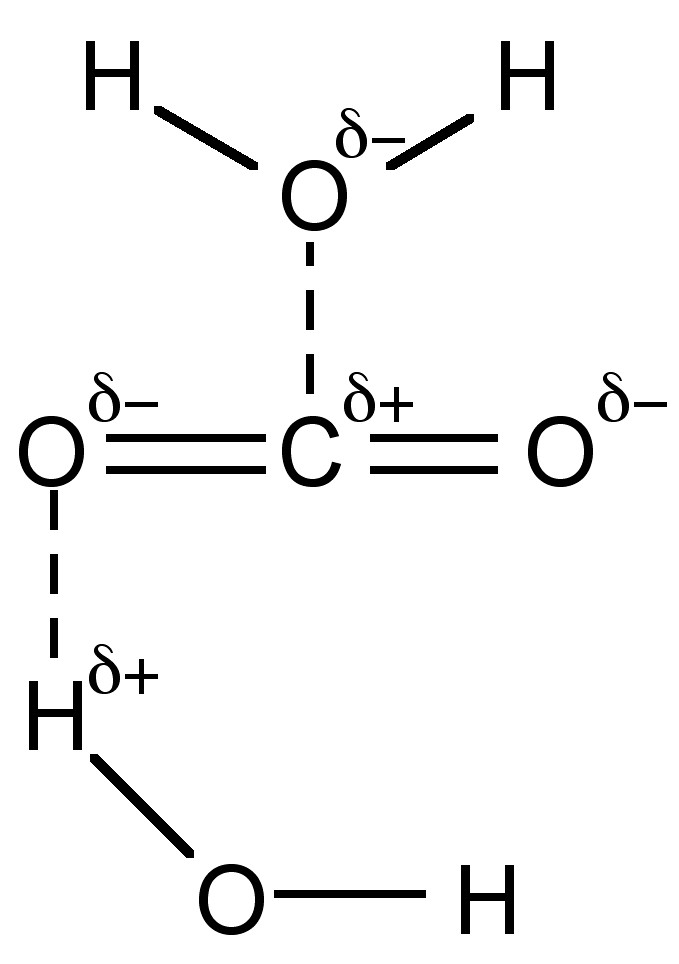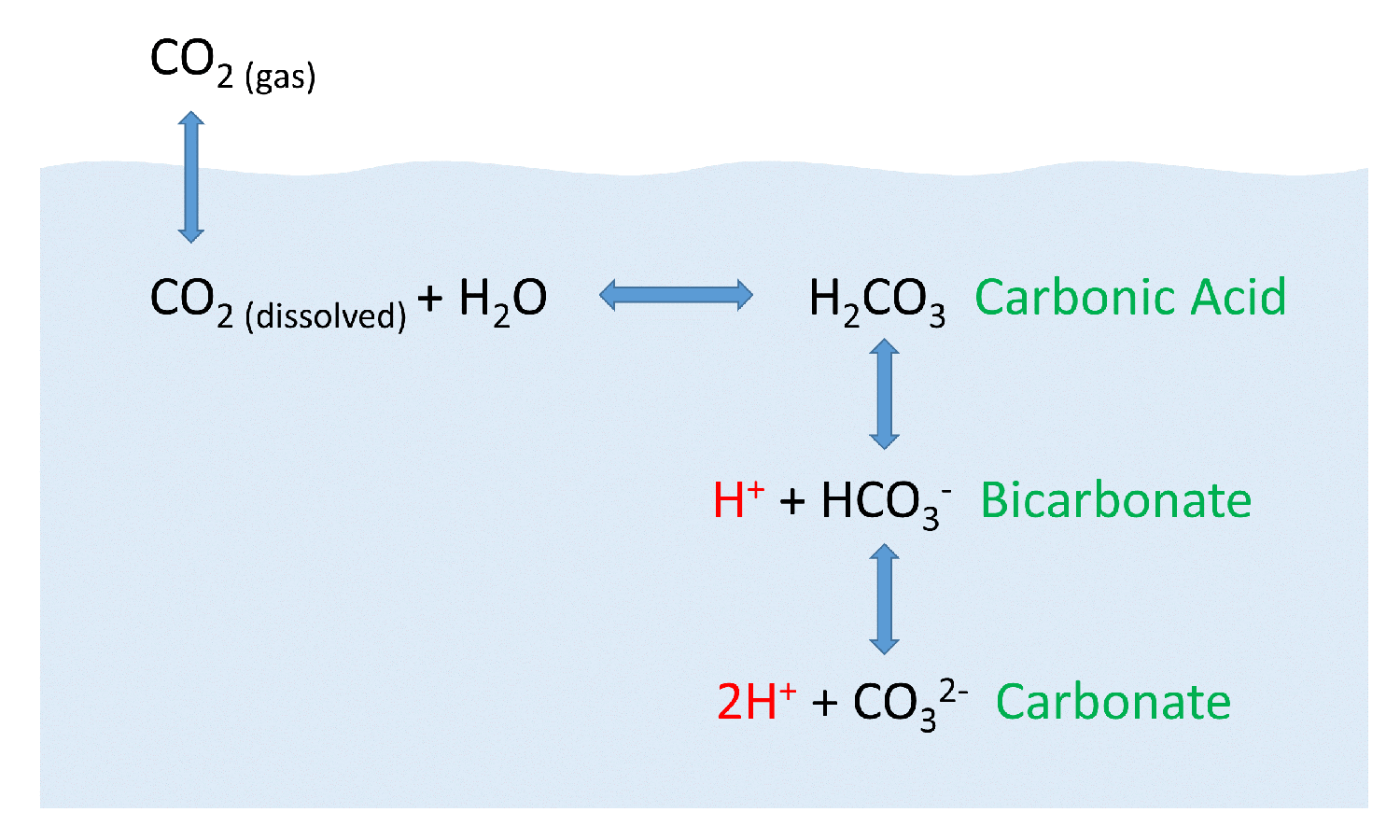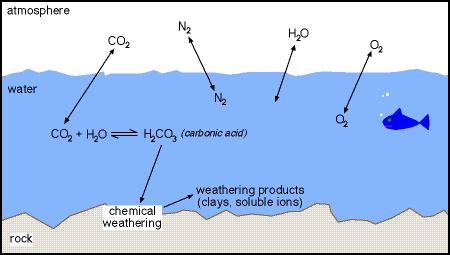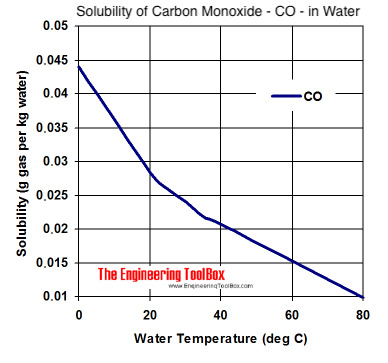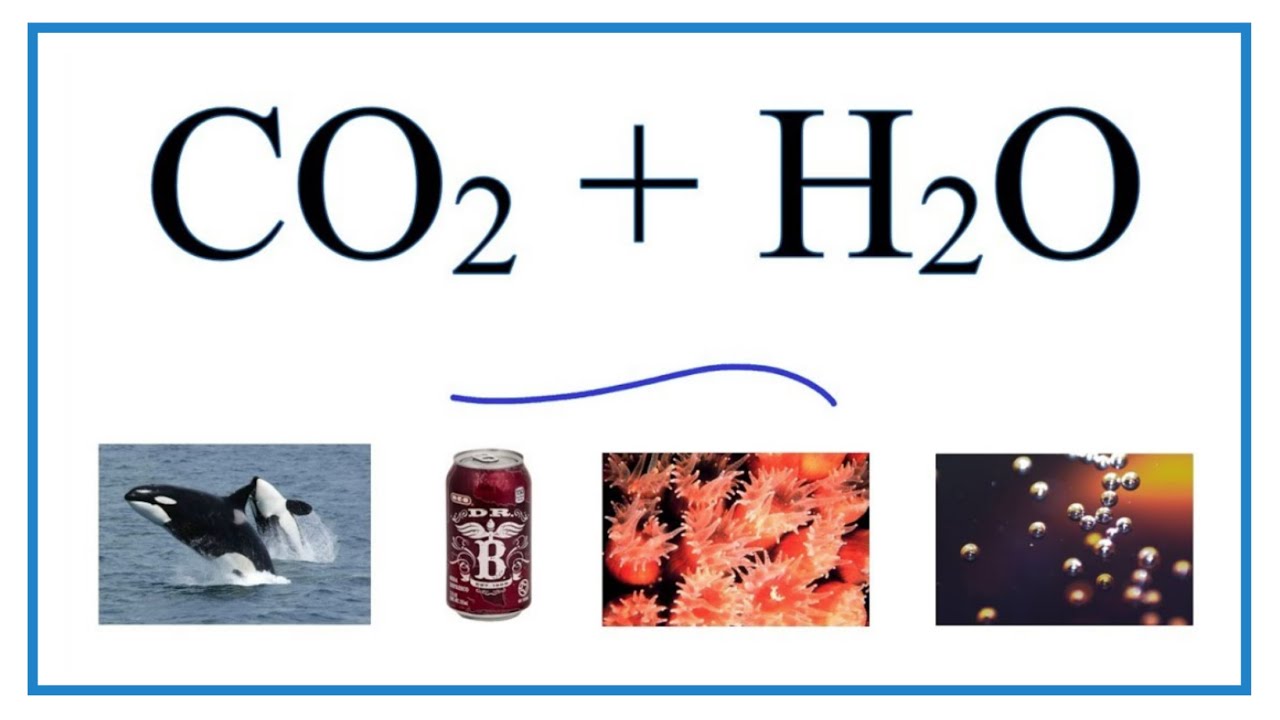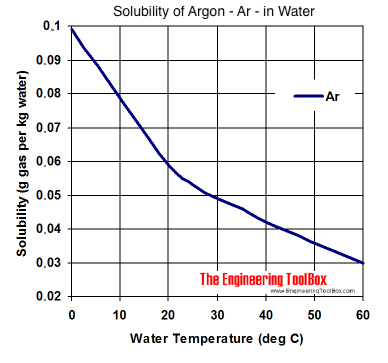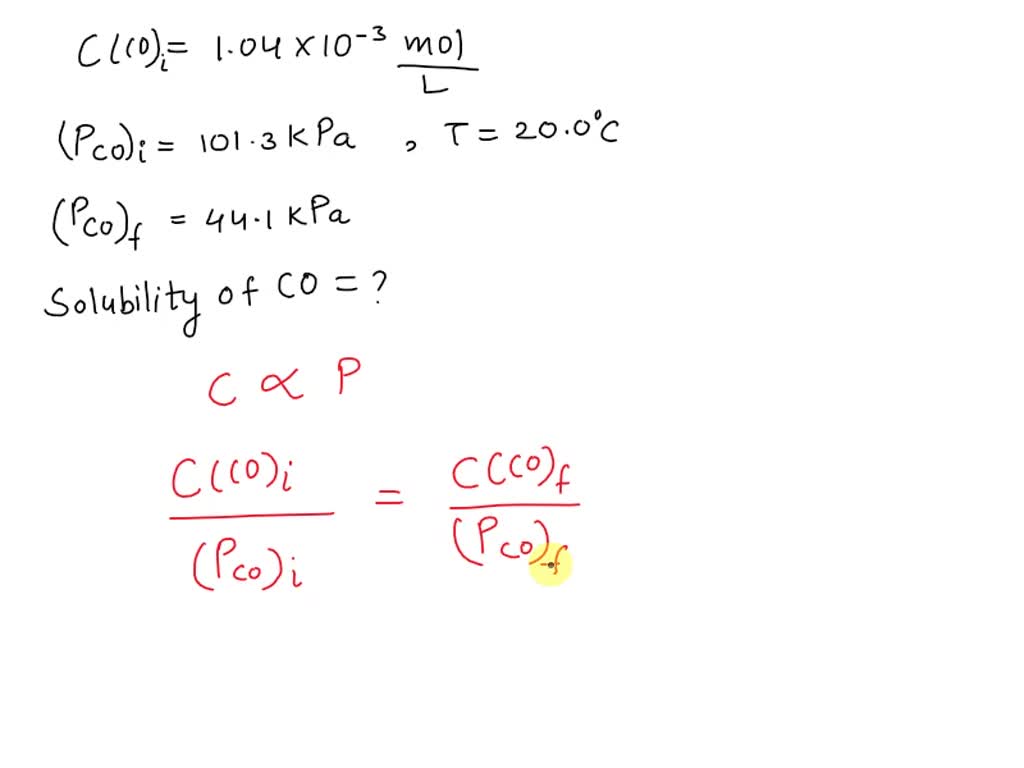
SOLVED: The concentration of dissolved carbon monoxide in water exposed to gaseous carbon monoxide at a partial pressure of 101.3 kPa at 20.0∘C is 1.04×10−3molL. Use Henry's law to determine the solubility

Allosteric Regulation in Carbon Monoxide (CO) Release: Anion Responsive CO-Releasing Molecule (CORM) Derived from (Terpyridine)phenol Manganese Tricarbonyl Complex with Colorimetric and Fluorescence Monitoring | Inorganic Chemistry

Electrochemical Carbon Monoxide Reduction on Polycrystalline Copper: Effects of Potential, Pressure, and pH on Selectivity toward Multicarbon and Oxygenated Products | ACS Catalysis

Carbon Dioxide in Water Solubility & Reaction | Is CO2 Soluble in Water? - Video & Lesson Transcript | Study.com

why is methane gas solubility in water higher than carbon monoxide solubility in water? - Chemistry Stack Exchange
![PDF] Solubility of hidrogen and carbon monoxide in water and some organic solvents | Semantic Scholar PDF] Solubility of hidrogen and carbon monoxide in water and some organic solvents | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/53e0b65259c80d553fe7dbb1371573c0d2fa02be/2-Table1-1.png)