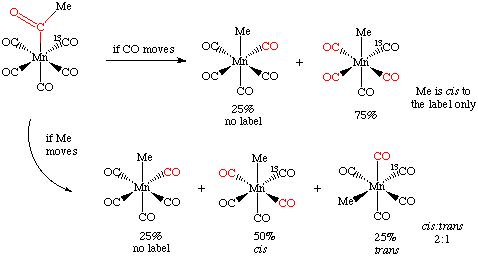
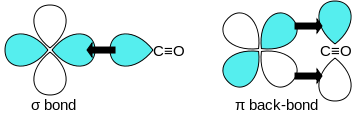
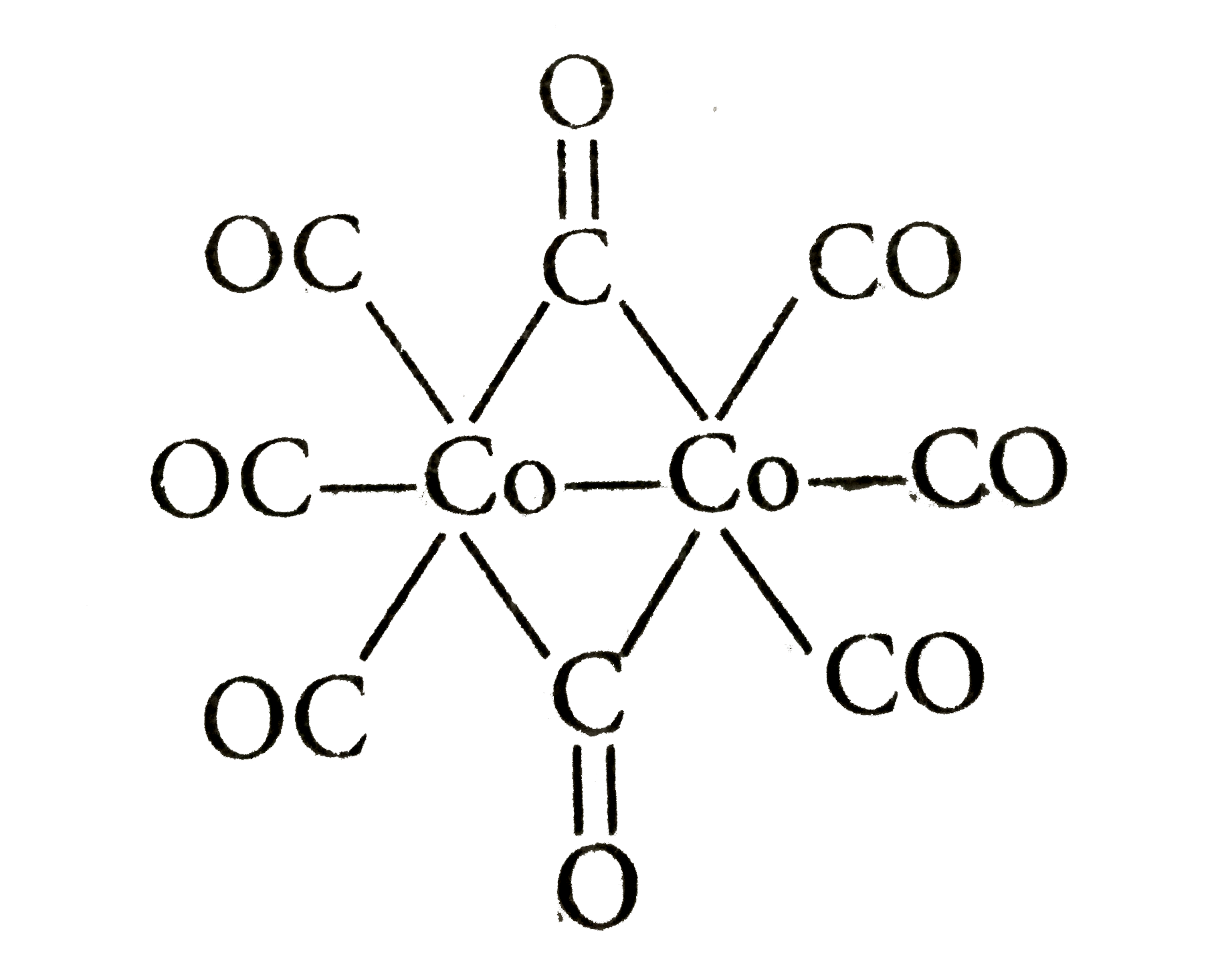
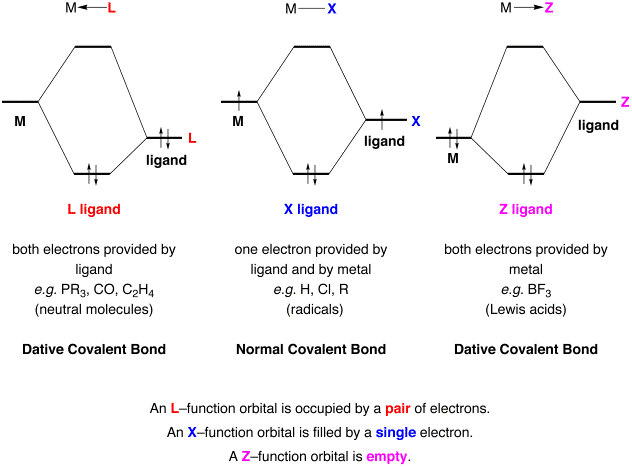
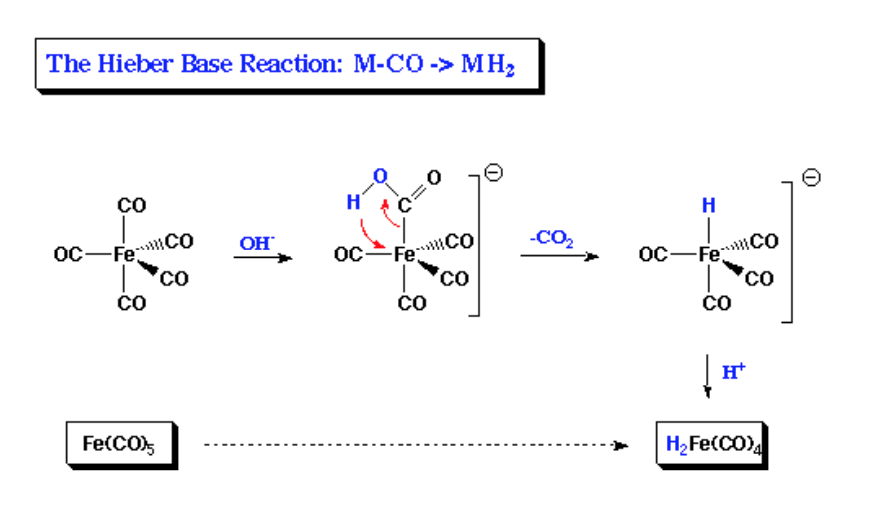
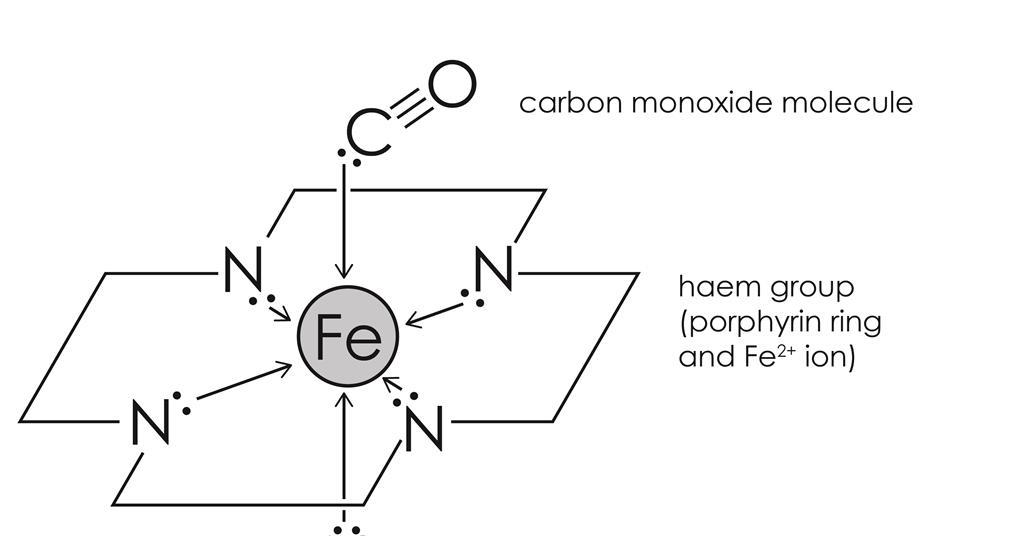
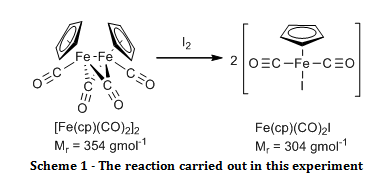
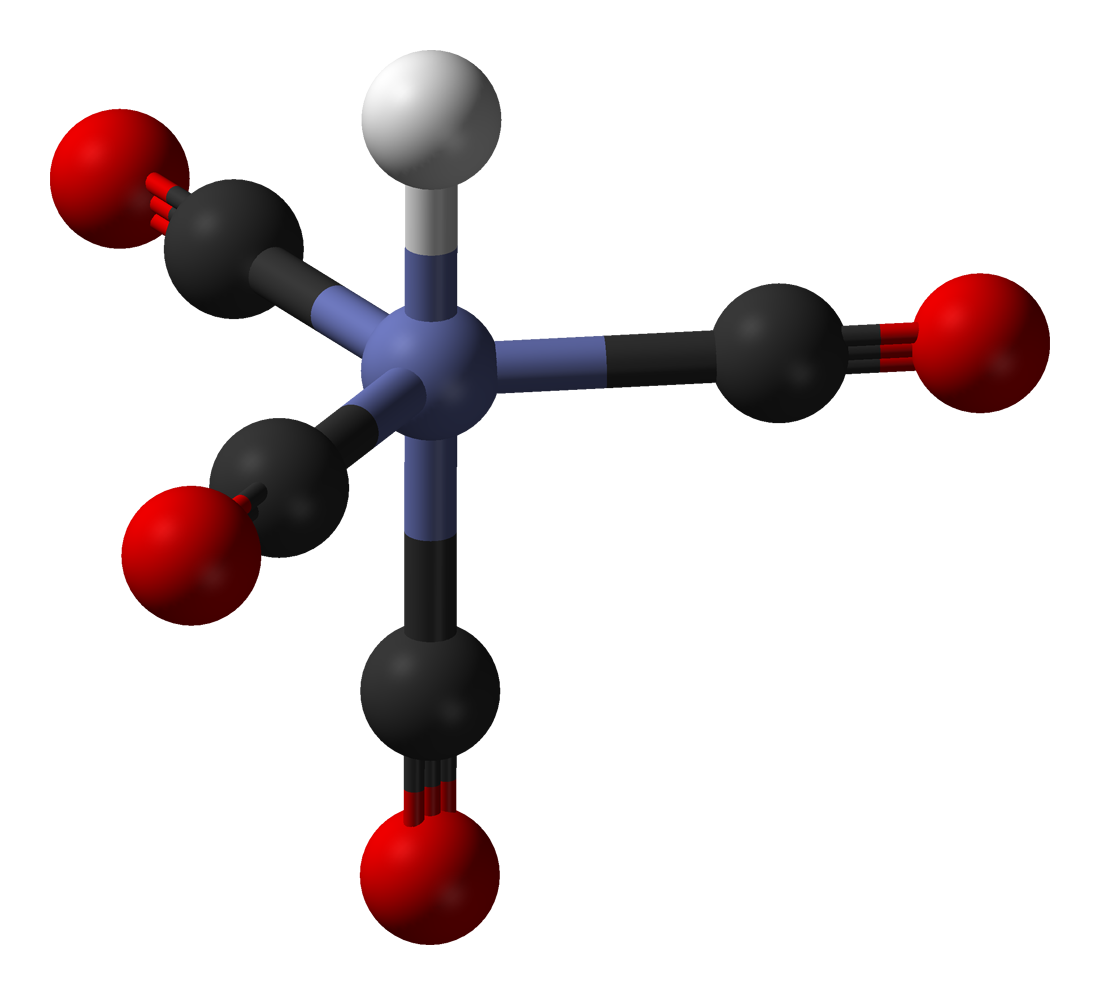
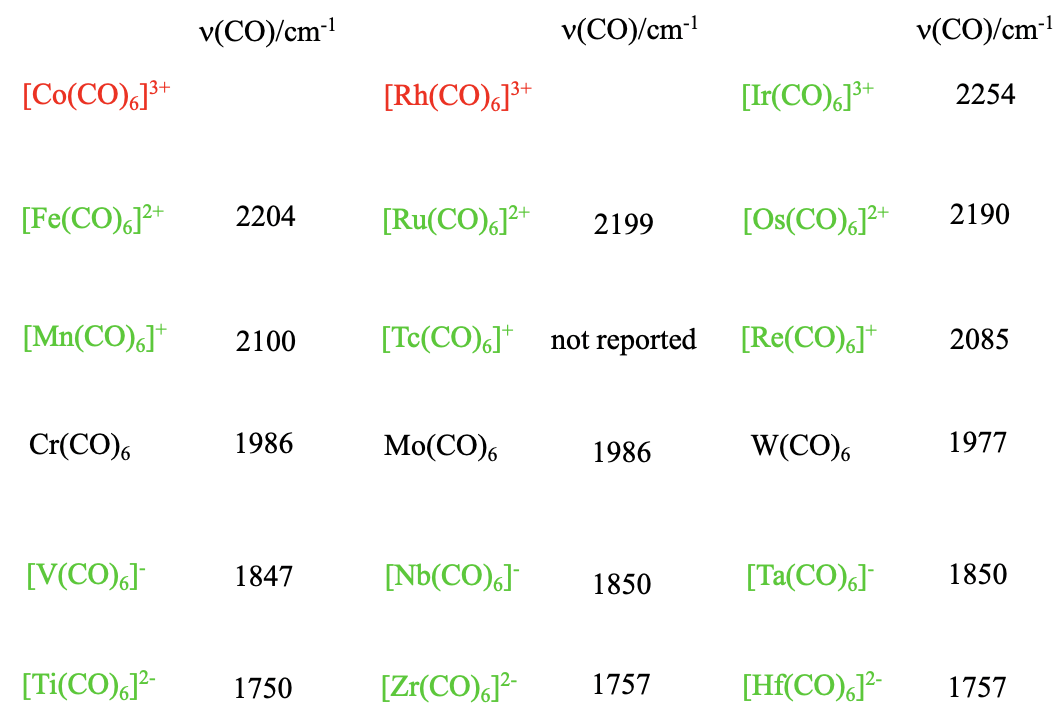Structure and Bonding of the Transition-Metal Carbonyl Complexes M(CO)5L (M = Cr, Mo, W) and M(CO)3L (M = Ni, Pd, Pt; L = CO, SiO, CS, N2, NO+, CN-, NC-, HCCH, CCH2,

Room-Temperature Reversible Chemisorption of Carbon Monoxide on Nickel(0) Complexes | Journal of the American Chemical Society

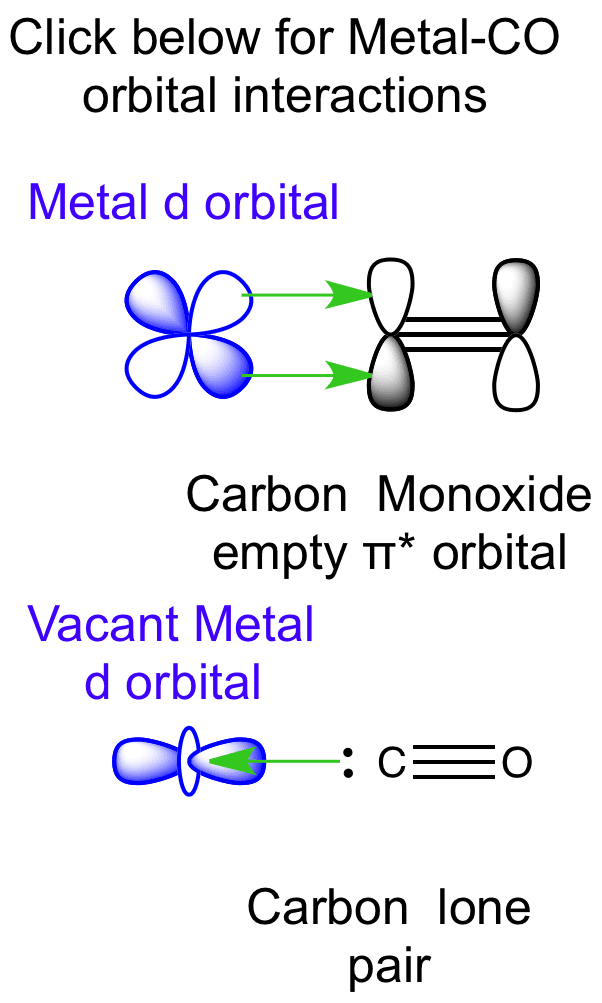
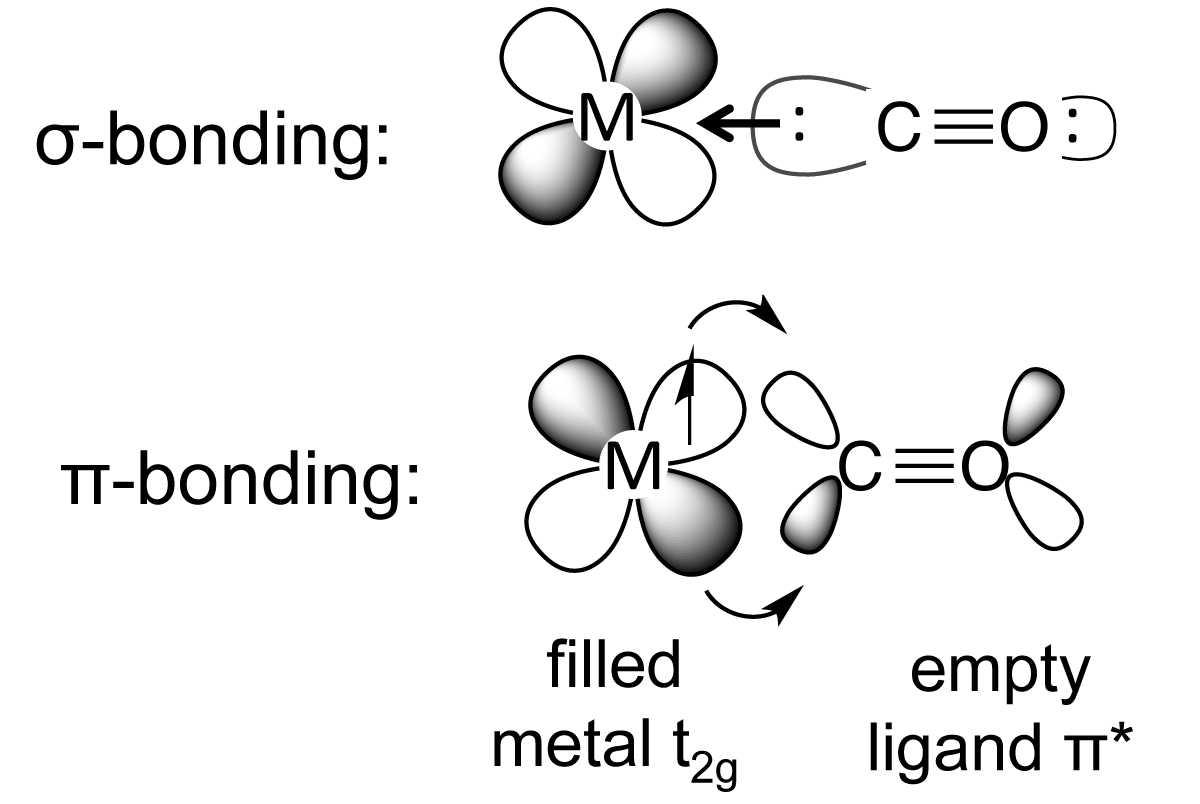
inorganic chemistry - Why CO is a stronger and more common ligand than N2? - Chemistry Stack Exchange

inorganic chemistry - Why CO is a stronger and more common ligand than N2? - Chemistry Stack Exchange

Room-Temperature Reversible Chemisorption of Carbon Monoxide on Nickel(0) Complexes | Journal of the American Chemical Society

Carbon Monoxide, Isocyanide, and Nitrile Complexes of Cationic, d0 Vanadium Bisimides: π-Back Bonding Derived from the π Symmetry, Bonding Metal Bisimido Ligand Orbitals | Inorganic Chemistry