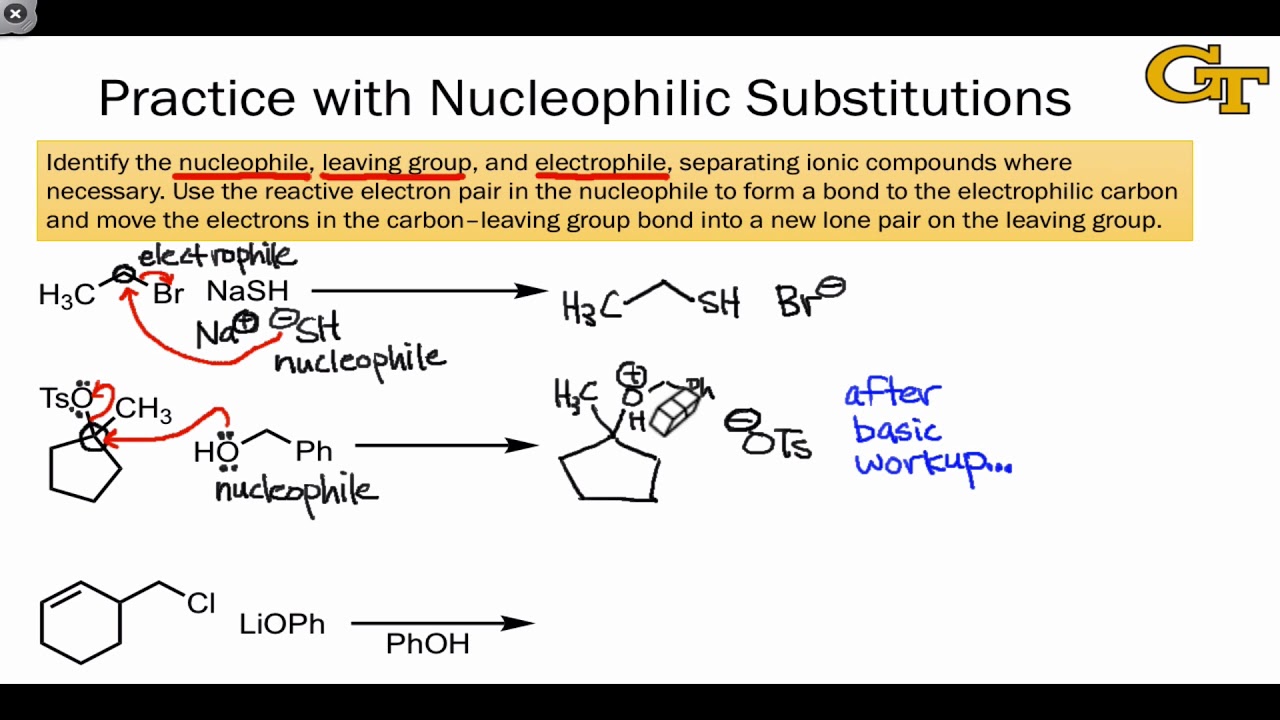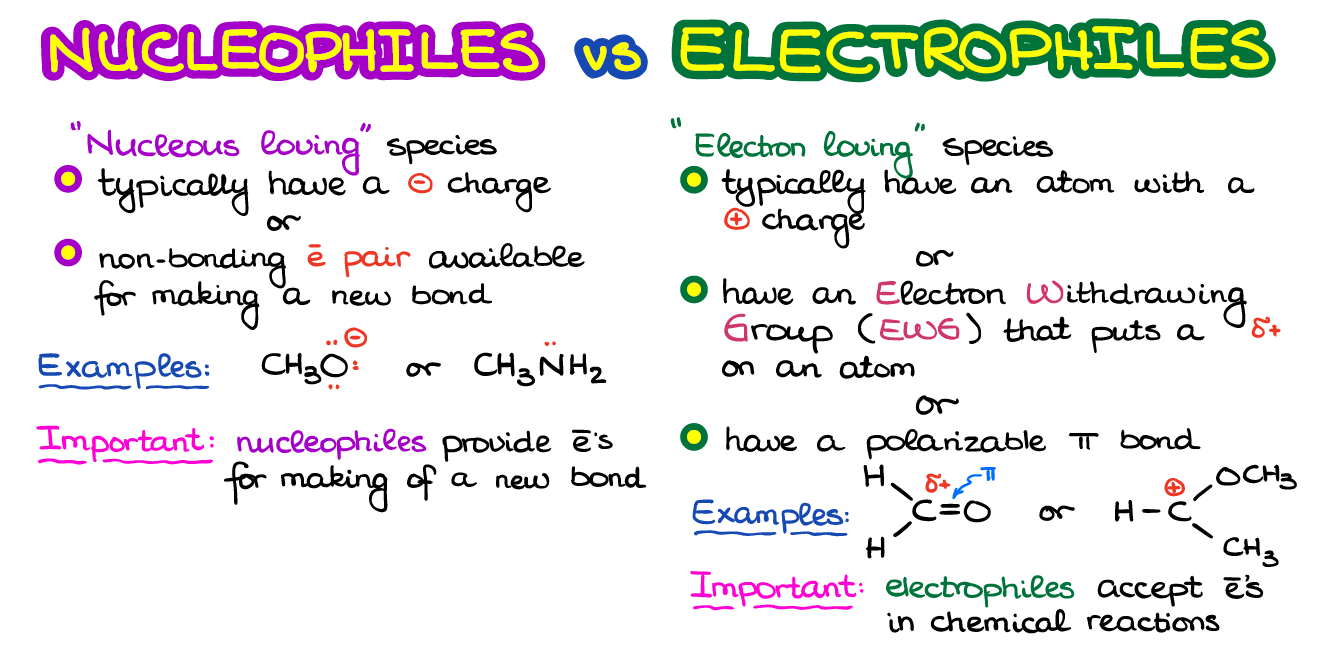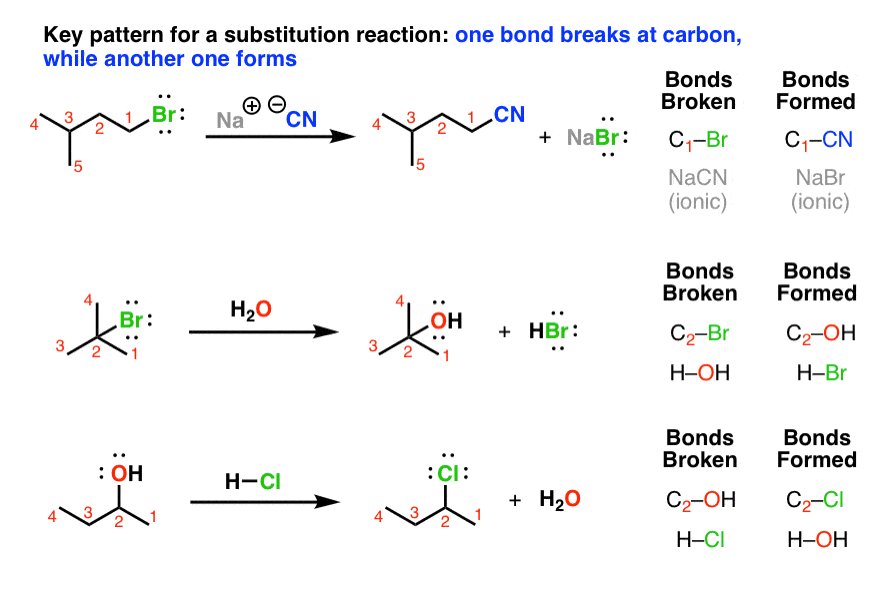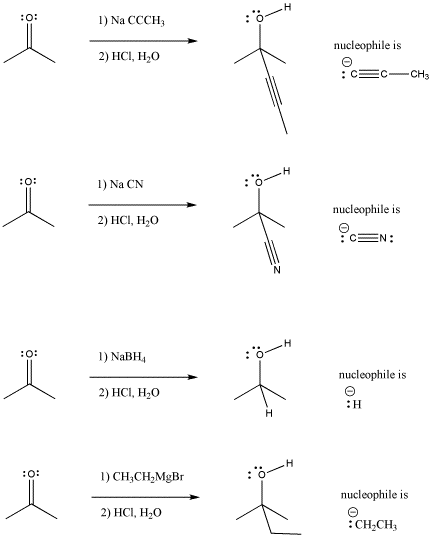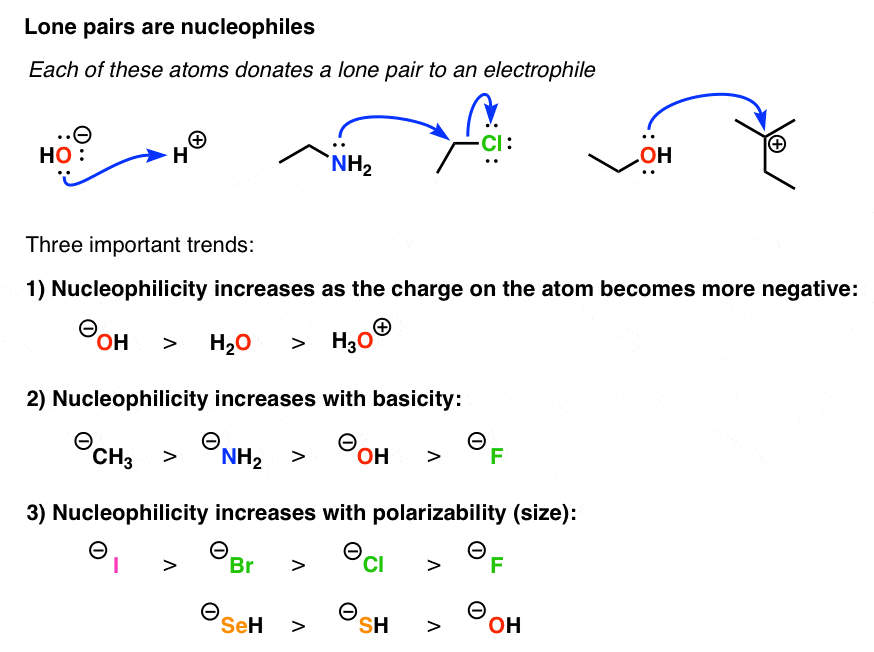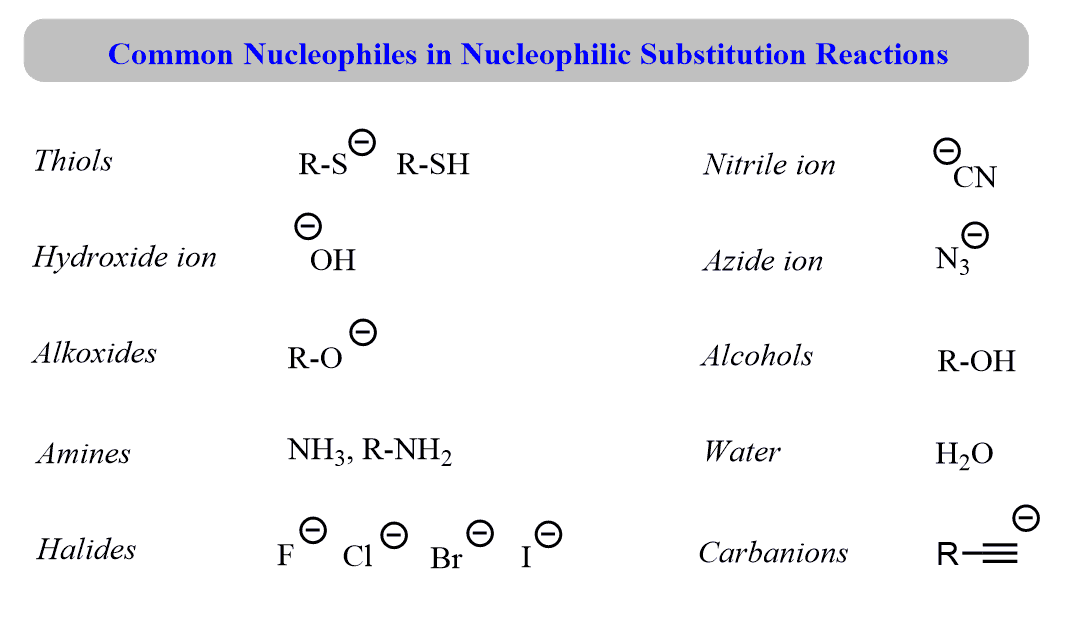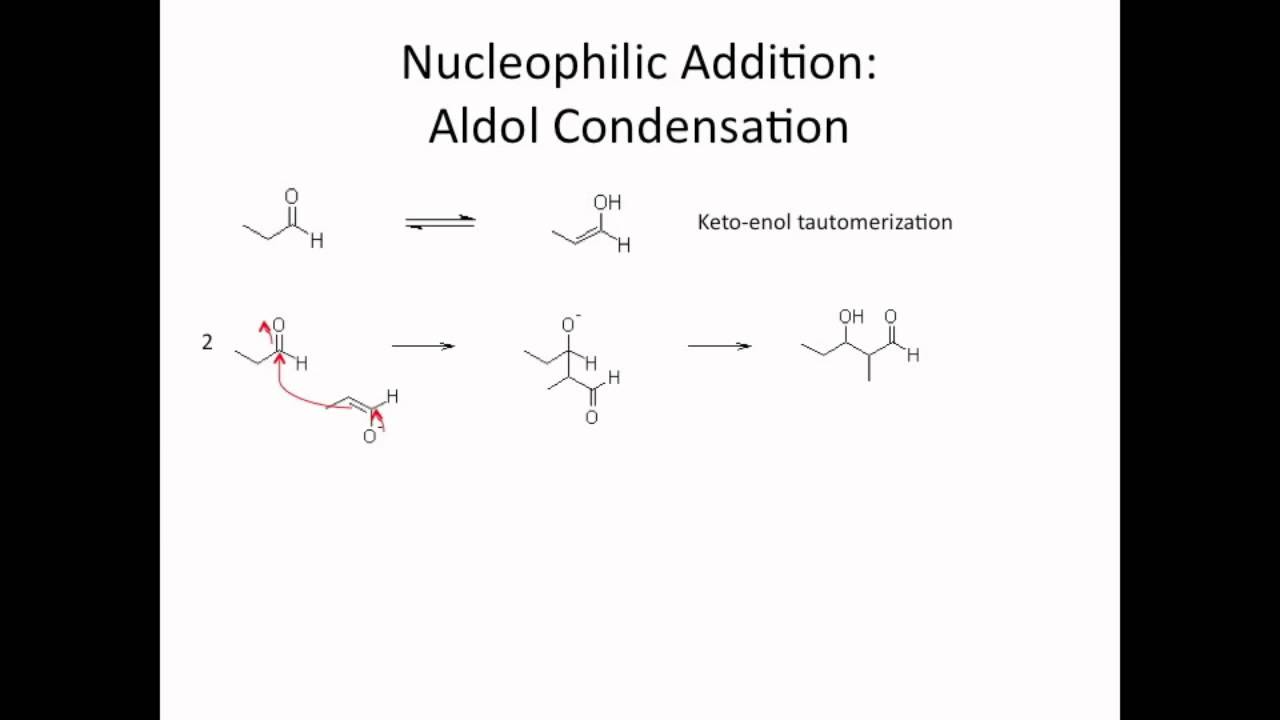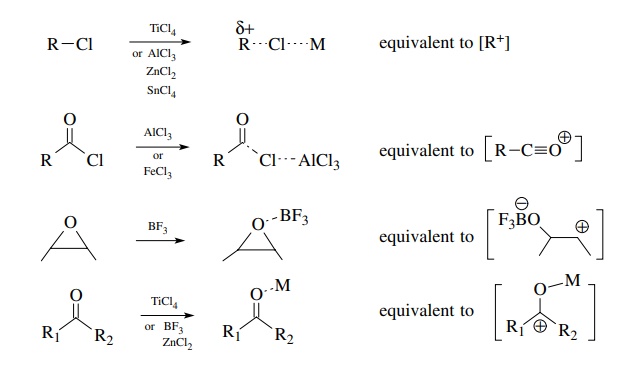
Neutral Carbon Nucleophiles - Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles | Organic Chemistry

Michael Addition of Soft Carbon Nucleophiles to Alkylidene Isoxazol-5-ones: A Divergent Entry to β-Branched Carbonyl Compounds. | Semantic Scholar
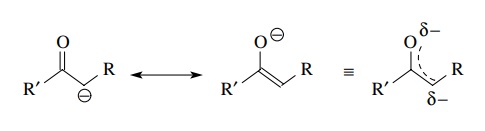
Nucleophilic Carbon - Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles | Organic Chemistry

Stereospecific and stereoconvergent nucleophilic substitution reactions at tertiary carbon centers - ScienceDirect

Carbon Nucleophiles in the Mitsunobu Reaction. Mono and Dialkylation of Bis(2,2,2-trifluorethyl) Malonates
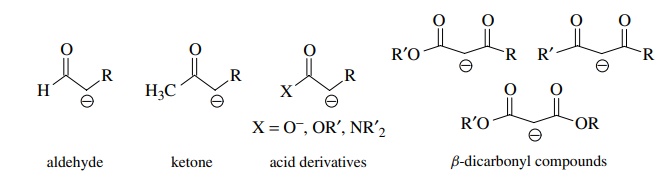


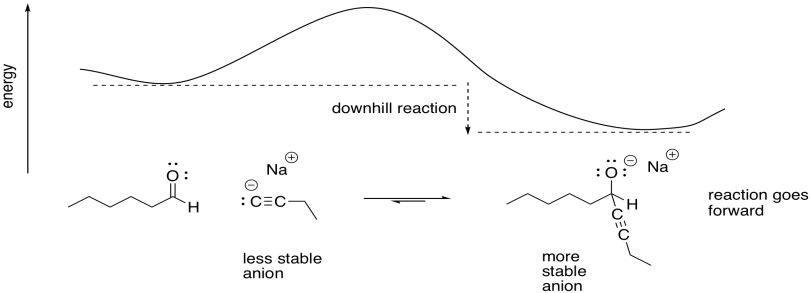

/chapter6/pages45and46/page45and46_files/hardsoftelec.png)