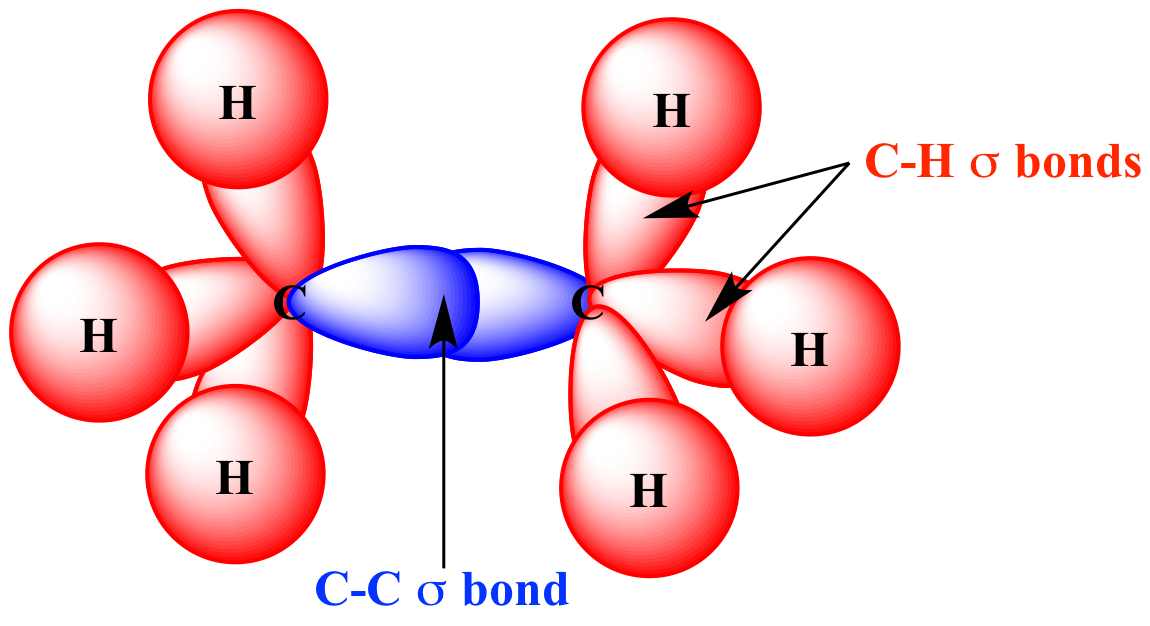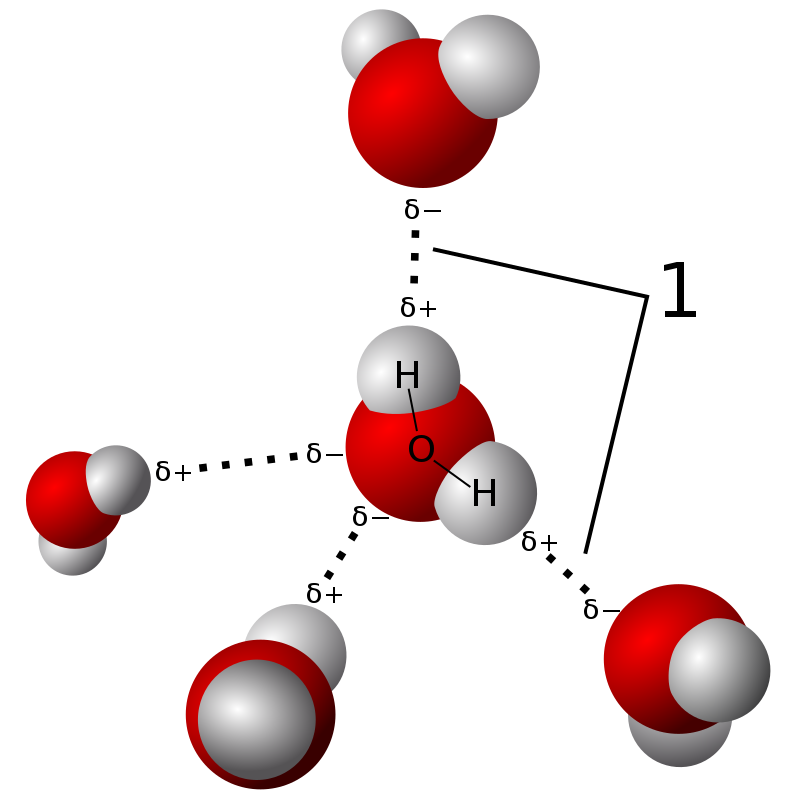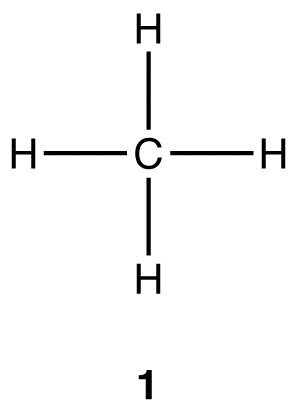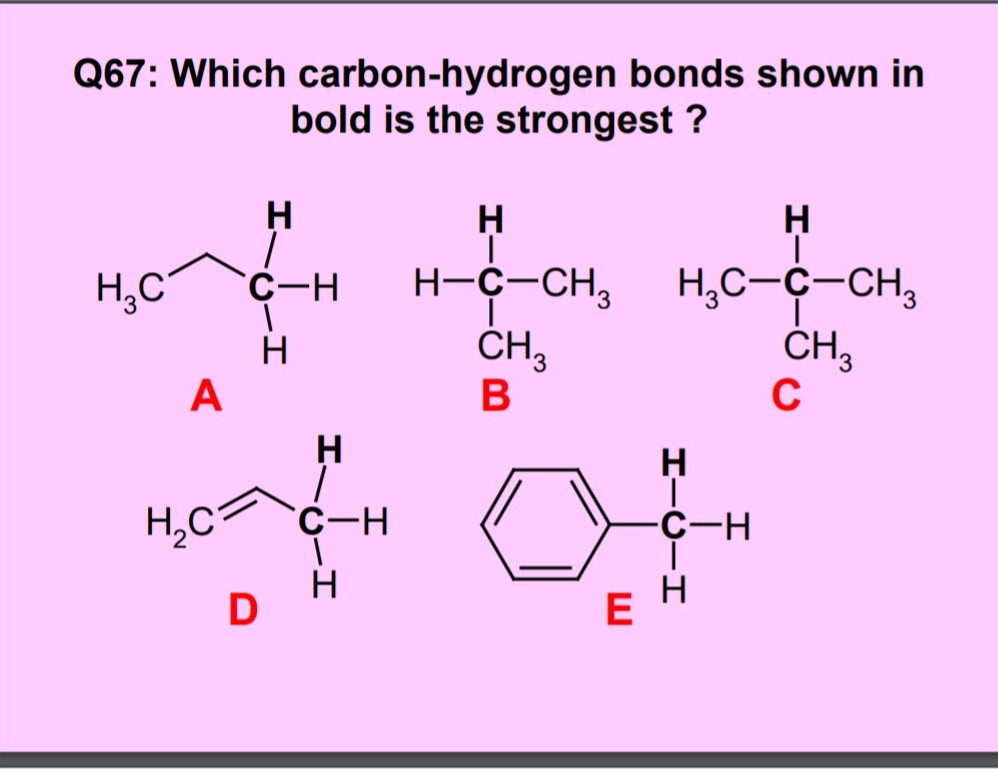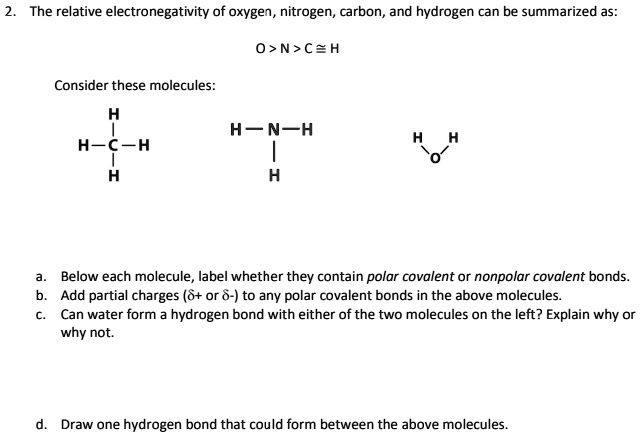
SOLVED: The relative electronegativity of oxygen, nitrogen, carbon, and hydrogen can be summarized as: 0>N>ceh Consider these molecules: HNH HCH Below each molecule, label whether they contain polar covalent or nonpolar covalent

Carbon–Hydrogen versus Nitrogen–Oxygen Bond Activation in Reactions of N-Oxide Derivatives of 2,2′-Bipyridine and 1,10-Phenanthroline with a Dimethylplatinum(II) Complex | Organometallics

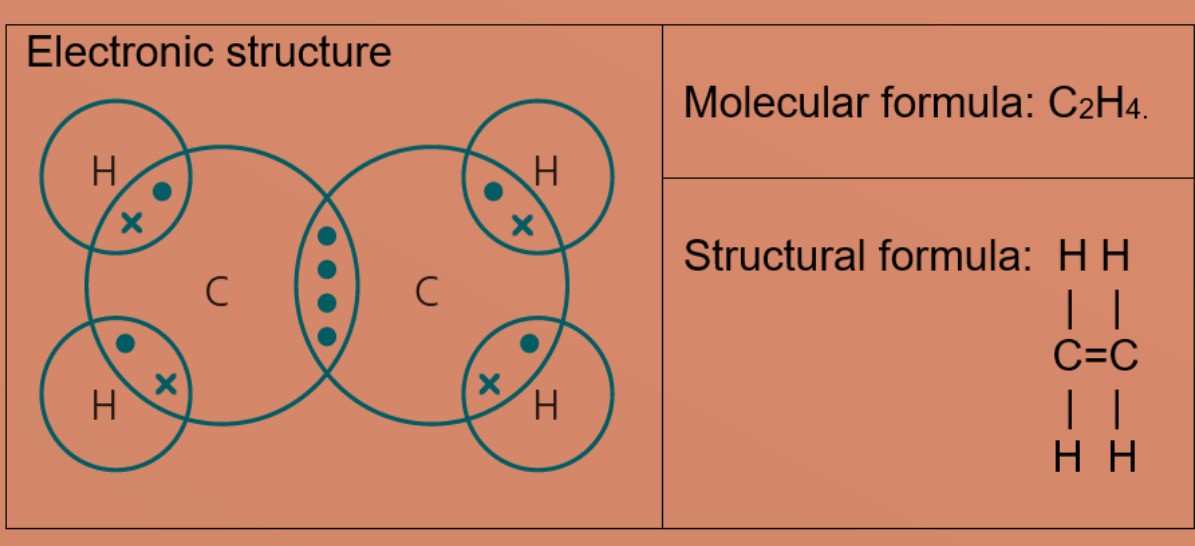
organic chemistry - Why are the hydrogen-carbon bonds bent in a graphical depiction of an alkene, but are straight horizontally and vertically in an alkane? - Chemistry Stack Exchange

electronic configuration - What happens to the 2s orbital in carbon-hydrogen bonds? - Chemistry Stack Exchange