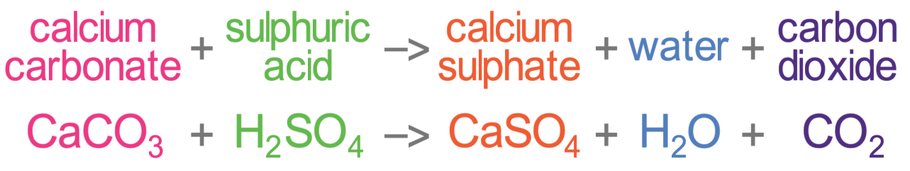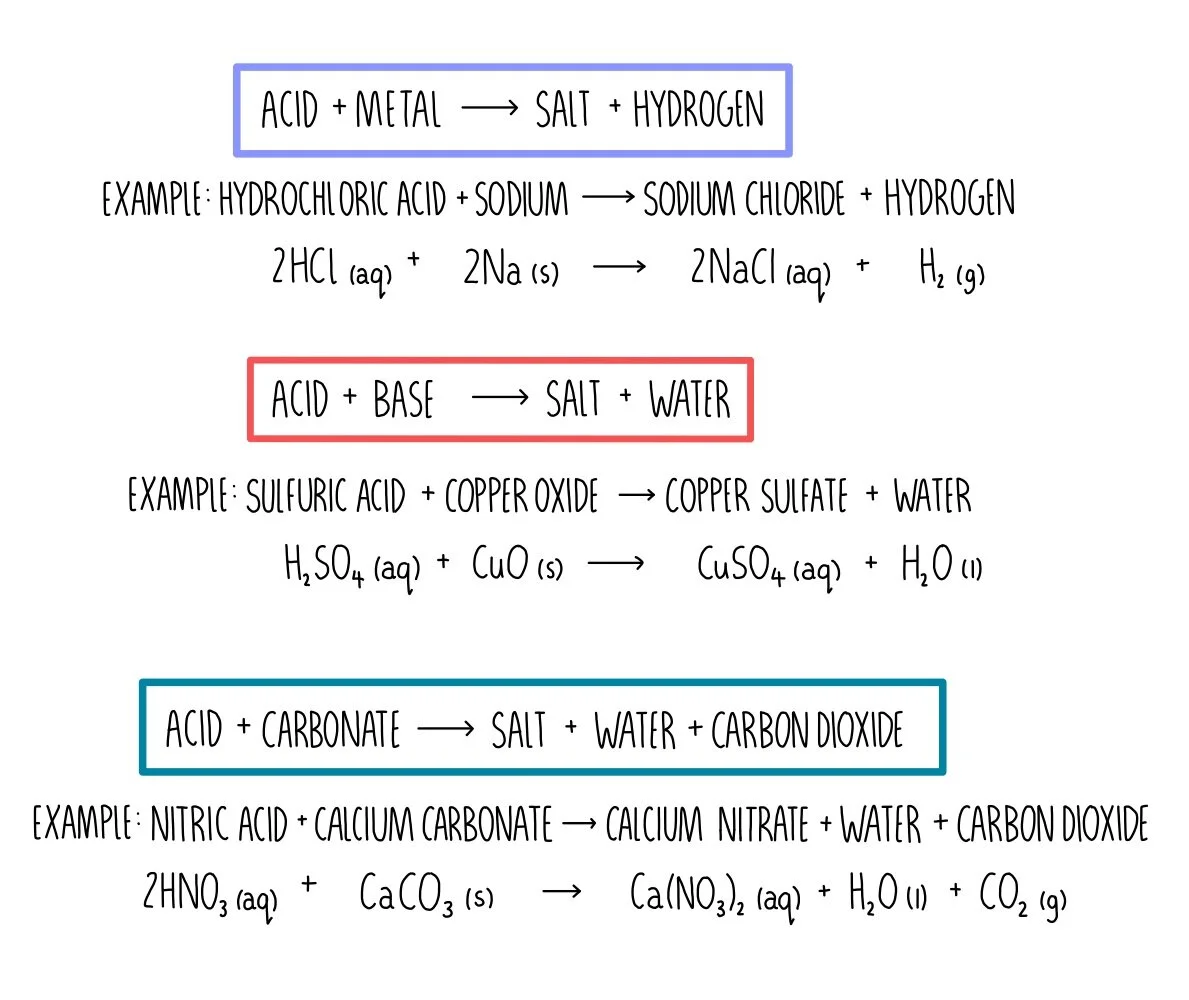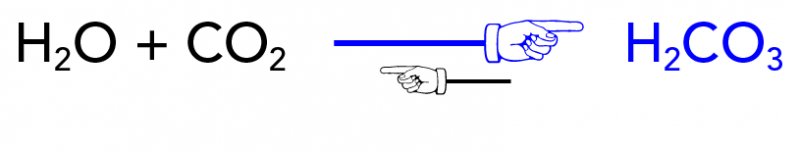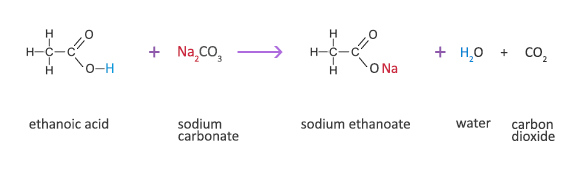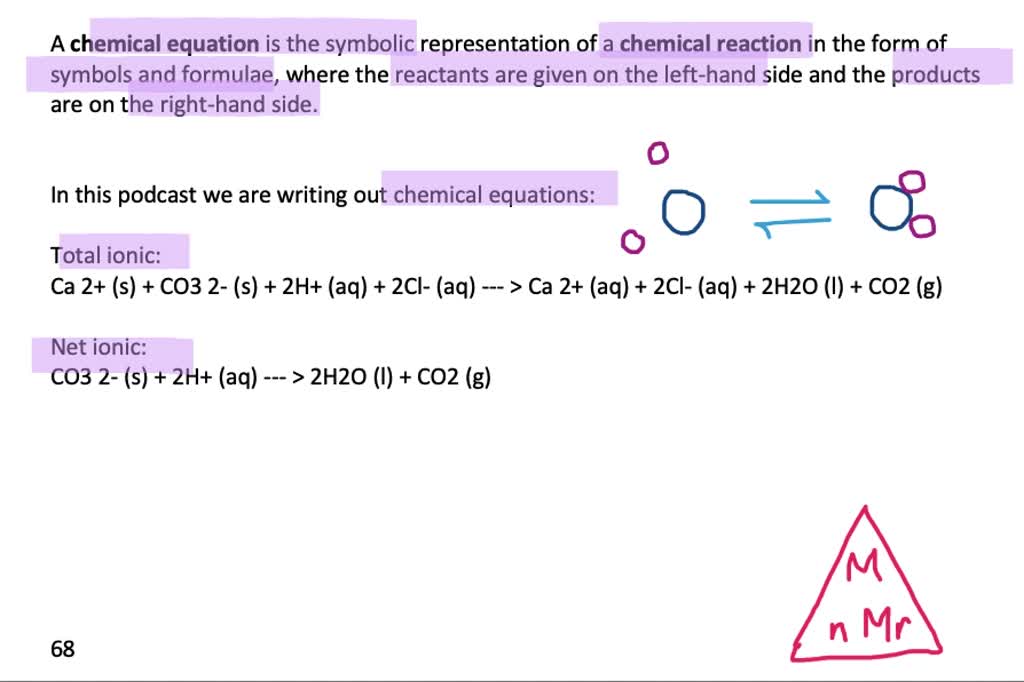
SOLVED:Limestone (calcium carbonate) is insoluble in water but dissolves when a hydrochloric acid solution is added. Write balanced total ionic and net ionic equations, showing hydrochloric acid as it actually exists in

physical chemistry - Which formula can be used to calculate the exact hydronium concentration present in sodium hydrogen carbonate solution? - Chemistry Stack Exchange

Why lime water turns milky due to formation of white precipitate of calcium carbonate , when carbon dioxide gas is passed through lime water ?

In a chemical reaction, when 14 g of sodium carbonate reacted with 10g of acetic acid (ethanoic acid), then it was observed that, 16.67 g of sodium acetate solution in water and

SOLVED: Sodium carbonate reacts with hydrochloric acid to form sodium chloride, water and carbon dioxide. A student mixed some sodium carbonate with an excess of hydrochloric acid in a beaker. The reaction
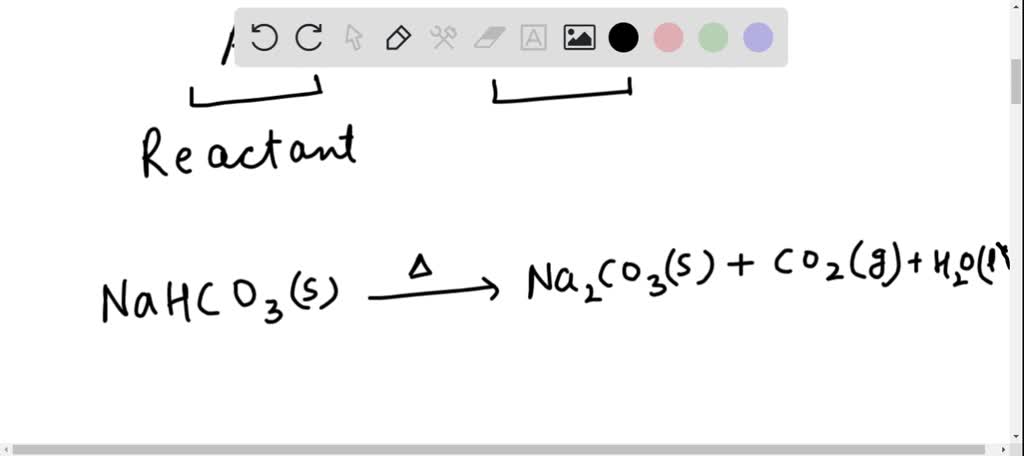
SOLVED: Write the balanced chemical reaction, including phase labels, for this reaction: "solid sodium bicarbonate decomposes into solid sodium carbonate, gaseous carbon dioxide, and liquid water."

How to Balance Ca(HCO3)2 = CaCO3 + CO2 + H2O (Decomposition of Calcium hydrogen carbonate) - YouTube