Exogenous citrate can react with carbonic acid to form citric acid and... | Download Scientific Diagram

SOLVED: Blood is buffered by the carbonic acid/bicarbonate buffer system: HzCO3 + HzO HCO3" HaOt If a relatively small amount of a strong acid were added to this buffer; which component of
Why is human blood basic (pH 7.4) if it contains a buffer of carbonic acid (H₂CO₃) and bicarbonate anion (HCO₃⁻) in order to maintain blood pH between 7.35 and 7.45? - Quora












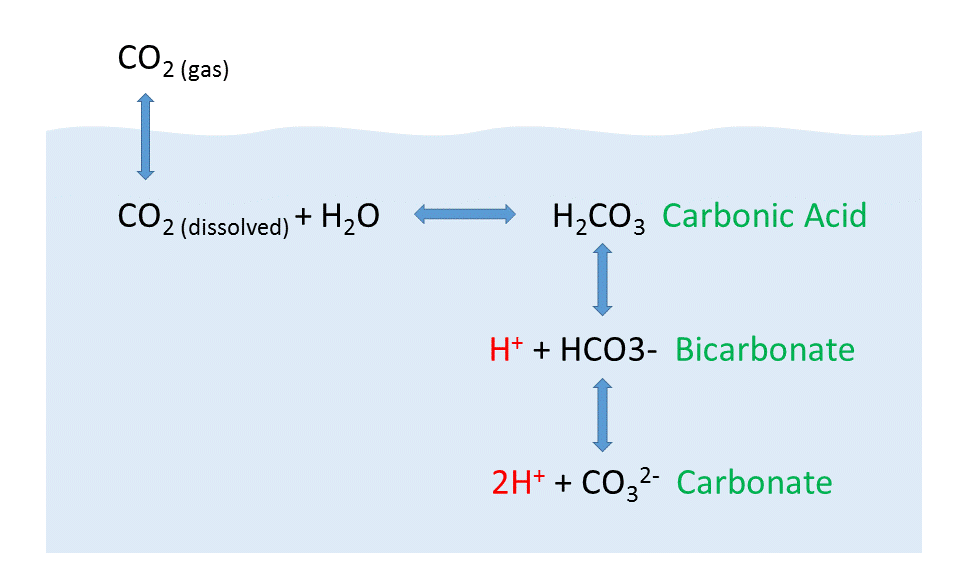
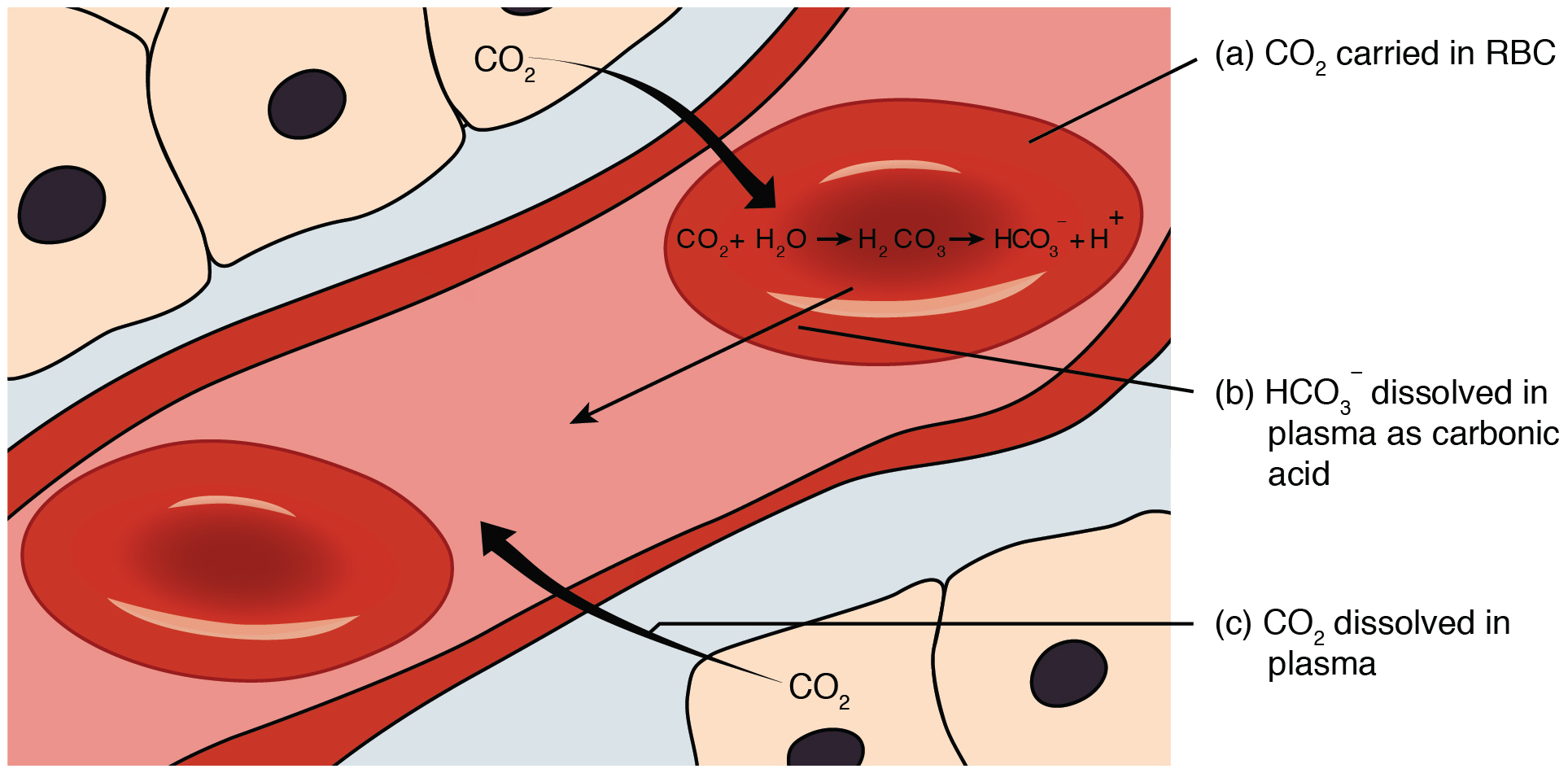
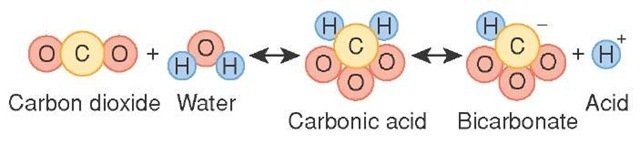



![ANSWERED] 2 CO H O H CO3 H HCO3 2H CO3 carbonic acid bicarbona... - Biology ANSWERED] 2 CO H O H CO3 H HCO3 2H CO3 carbonic acid bicarbona... - Biology](https://media.kunduz.com/media/sug-question-candidate/20230205051412057036-5005644.jpg)
