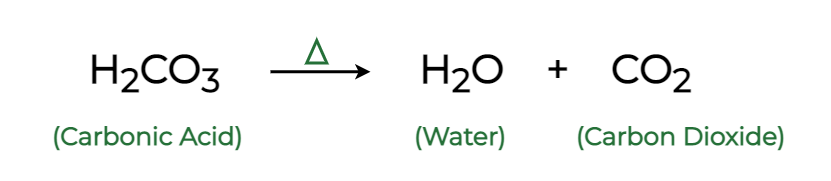
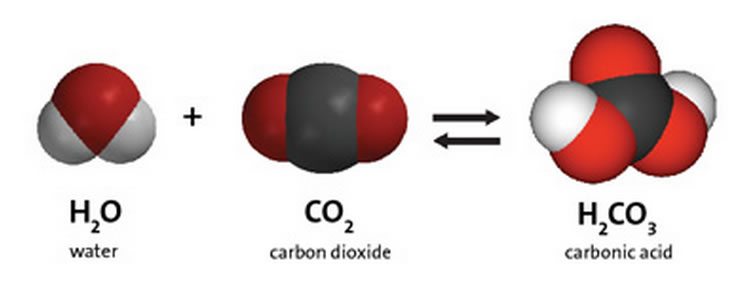
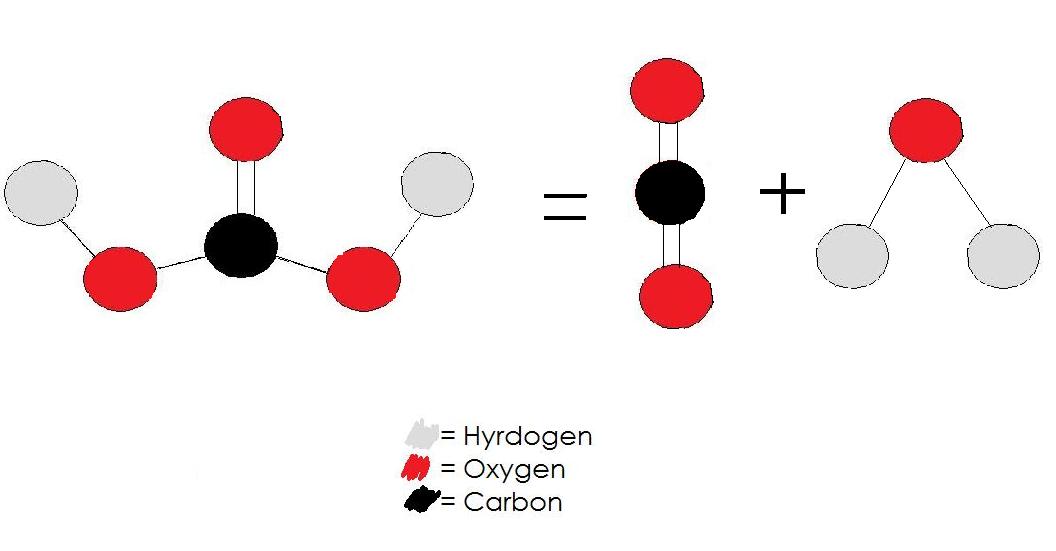
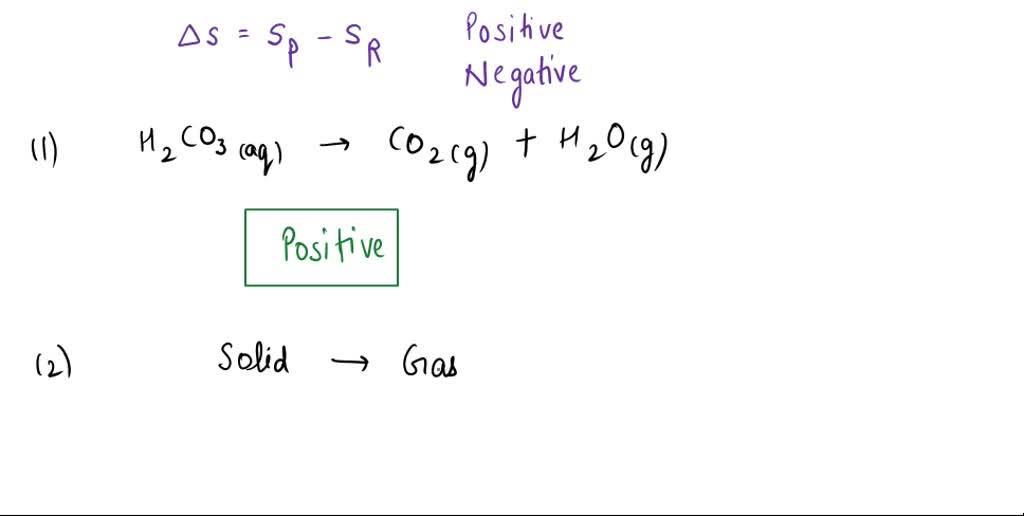H2CO3 → CO2 + H2O decomposition in the presence of H2O, HCOOH, CH3COOH, H2SO4 and HO2 radical: instability of the gas-phase H2
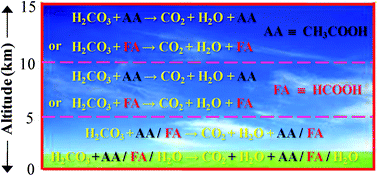
H2CO3 → CO2 + H2O decomposition in the presence of H2O, HCOOH, CH3COOH, H2SO4 and HO2 radical: instability of the gas-phase H2CO3 molecule in the troposphere and lower stratosphere - RSC Advances (
Visualization of the shortest routes for the decomposition of isolated... | Download Scientific Diagram

Carbonic acid formation in the atmosphere. Processing of mineral dust... | Download Scientific Diagram

Mechanistic insights into the dissociation and decomposition of carbonic acid in water via the hydroxide route: an ab initio metadynamics study. | Semantic Scholar

Protonated Carbonic Acid and Reactive Intermediates in the Acidic Decarboxylation of Indolecarboxylic Acids | The Journal of Organic Chemistry

Visualization of the shortest routes for the decomposition of isolated... | Download Scientific Diagram
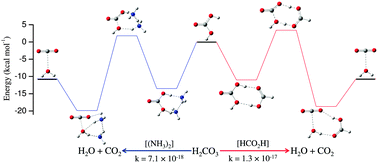
Ammonia as an efficient catalyst for decomposition of carbonic acid: a quantum chemical investigation - Physical Chemistry Chemical Physics (RSC Publishing)
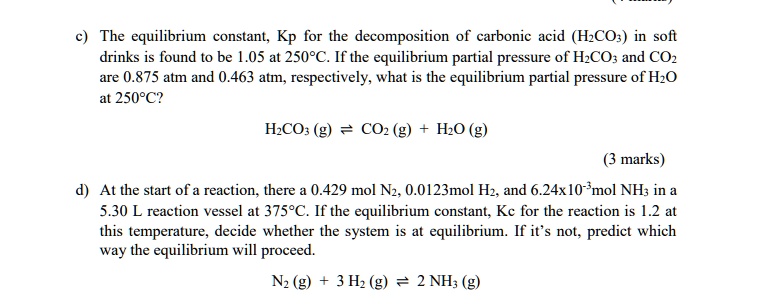
SOLVED: The equilibrium constant; Kp for the decomposition of carbonic acid (HzCOs) in sofit drinks is found to be 1.05 at 2508C. If the equilibrium partial pressure of H-COz and COz are