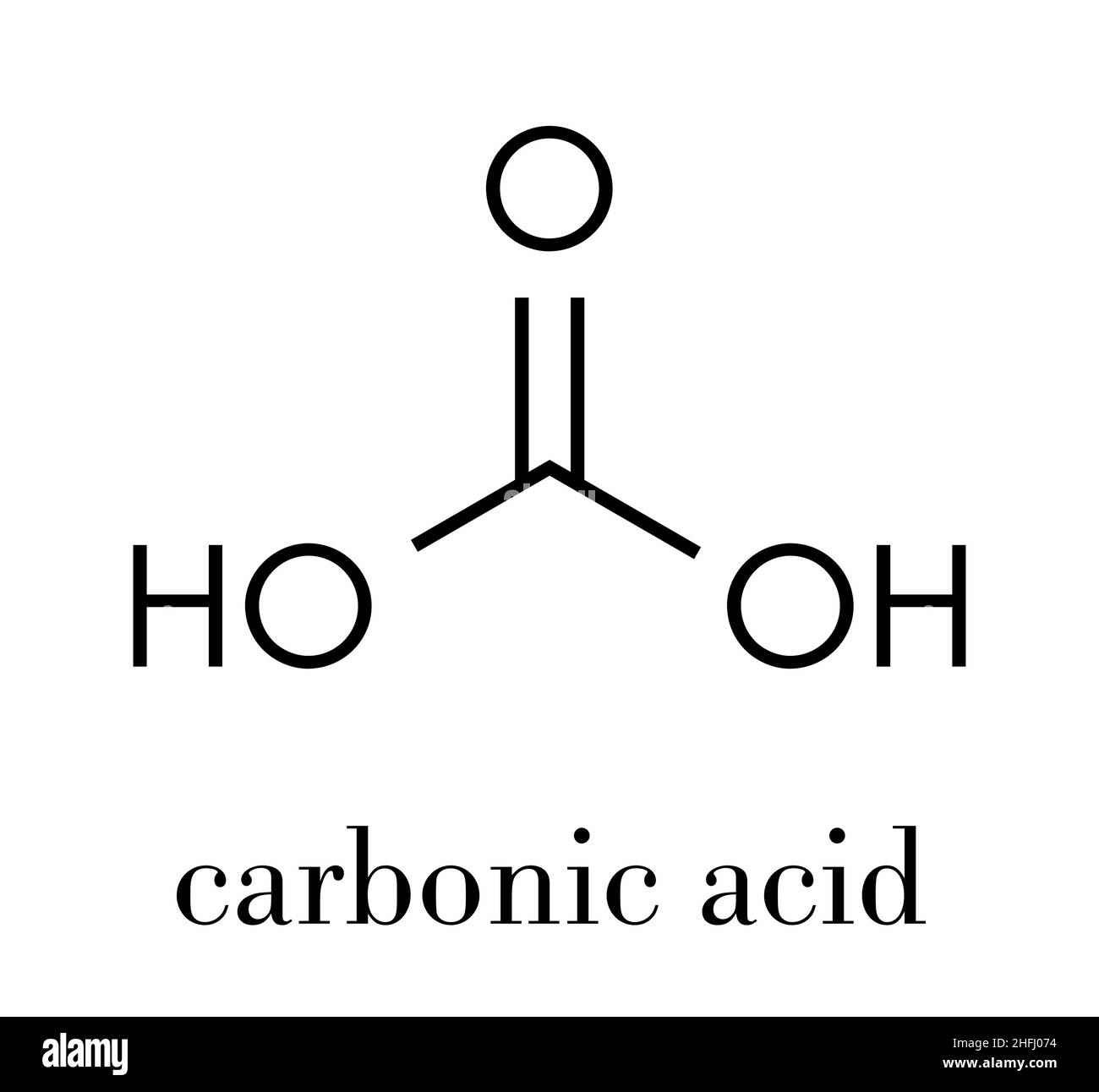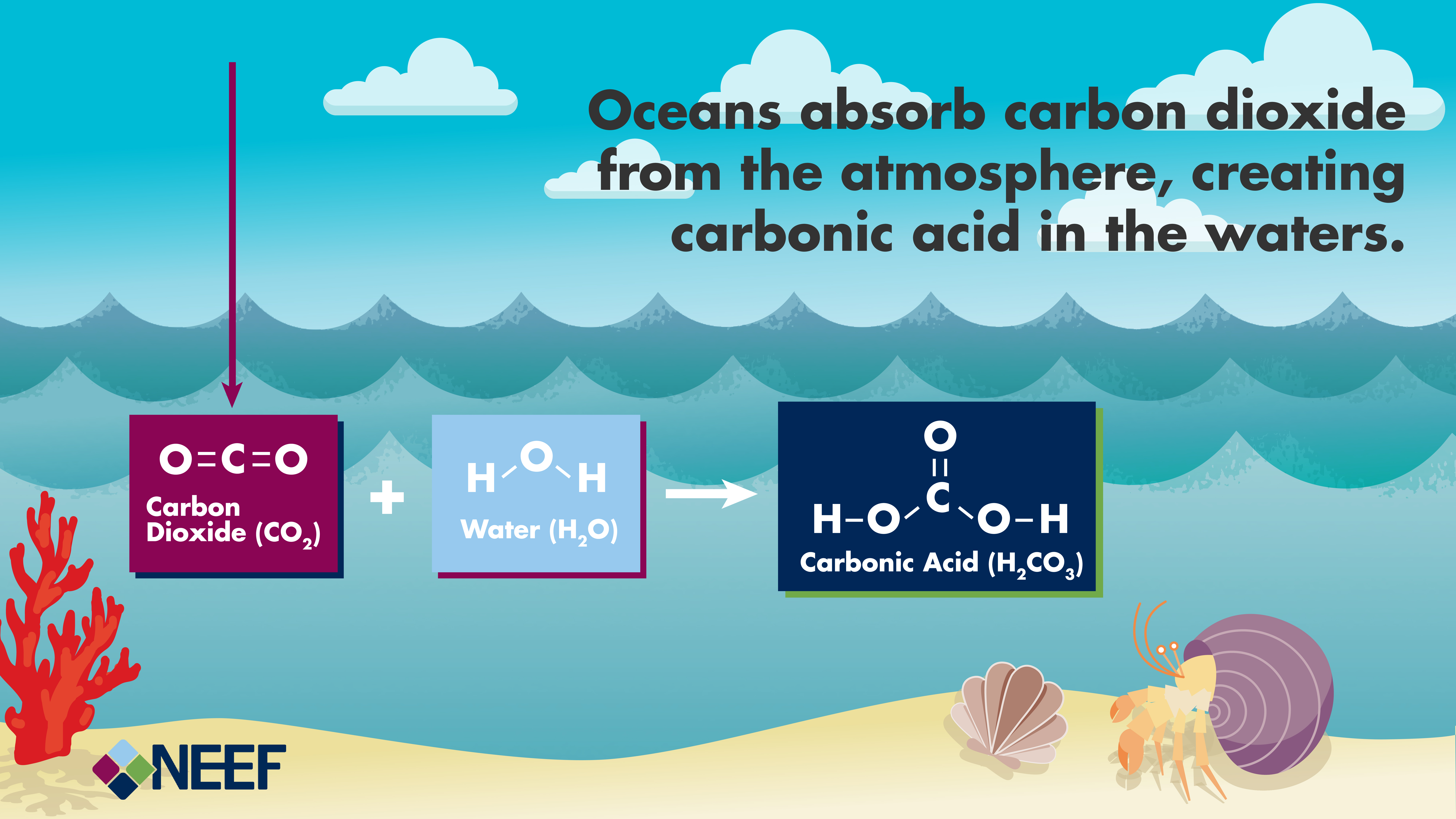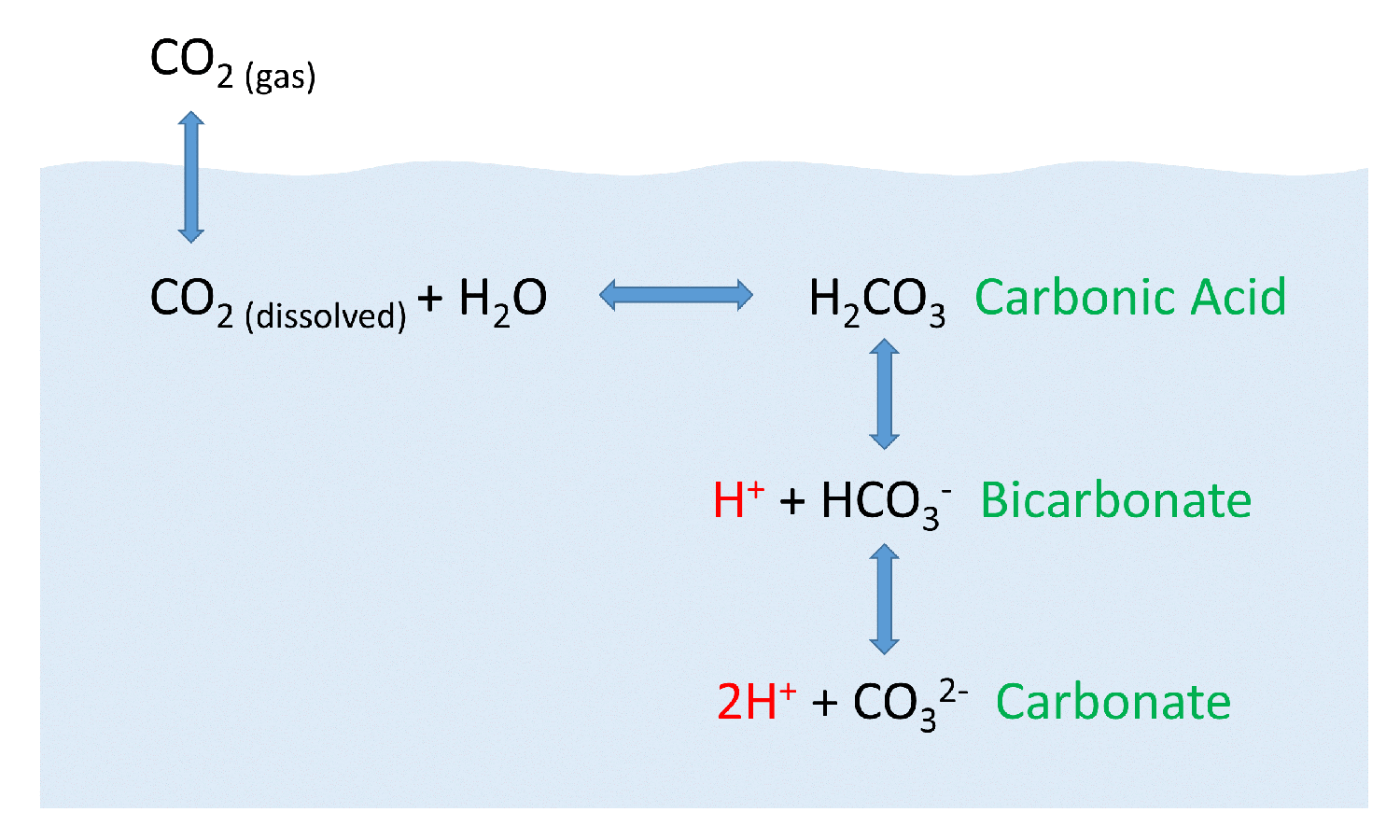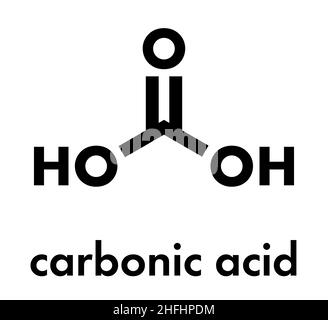
Carbonic acid molecule. Formed when carbon dioxide is dissolved in water (carbonated water). Skeletal formula Stock Vector Image & Art - Alamy
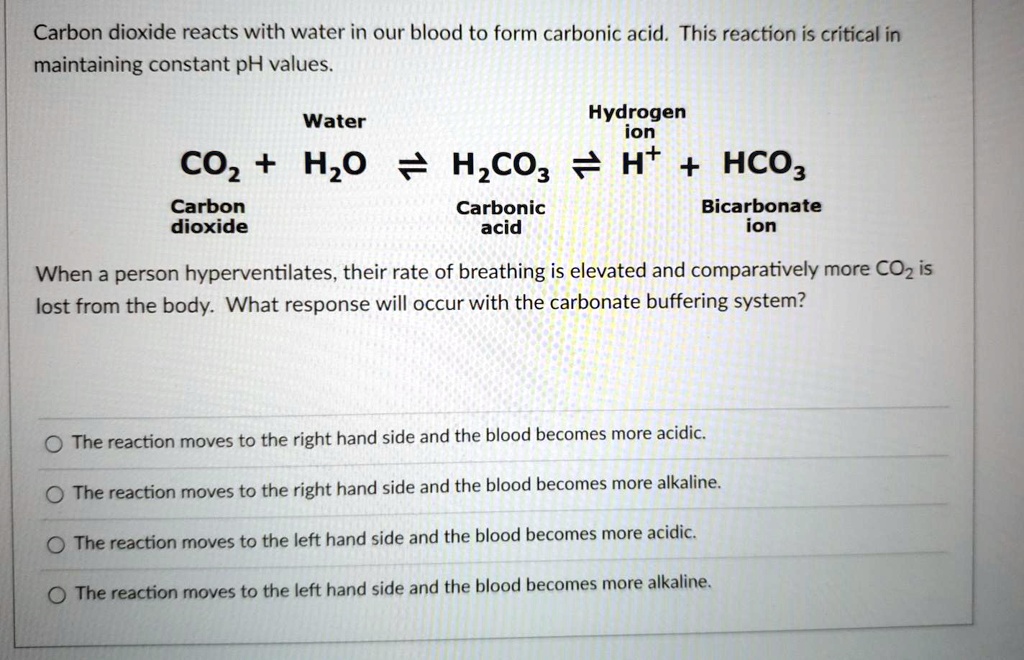
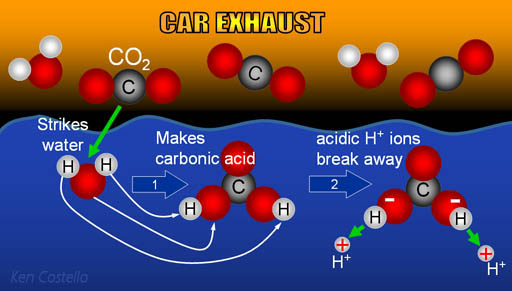
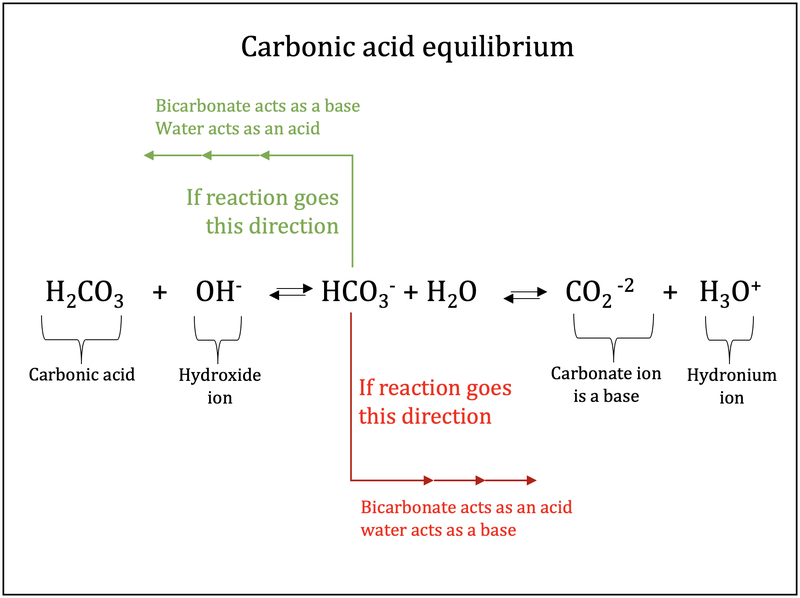
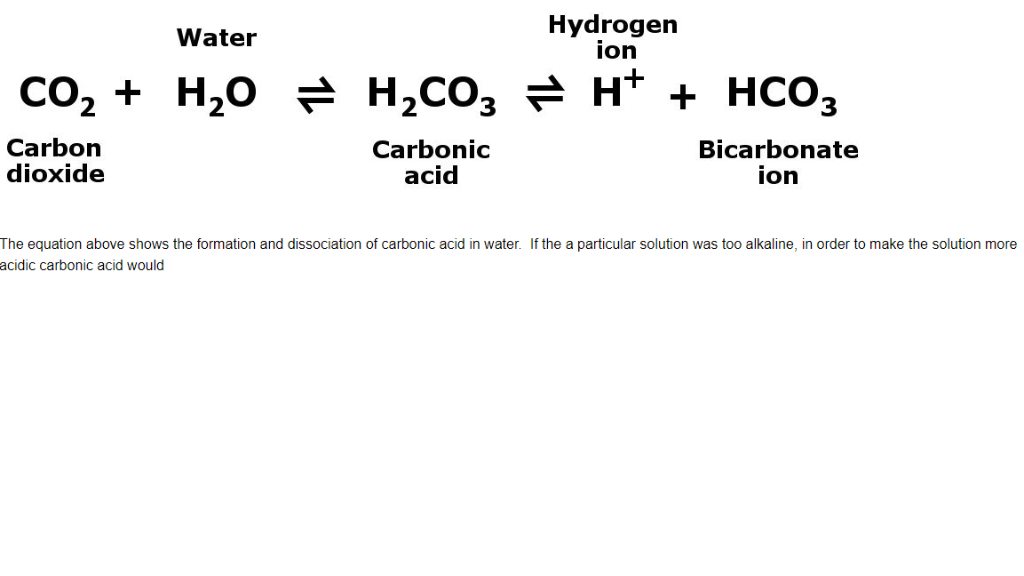
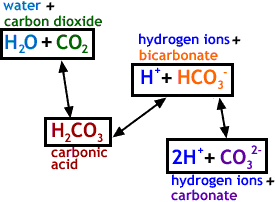
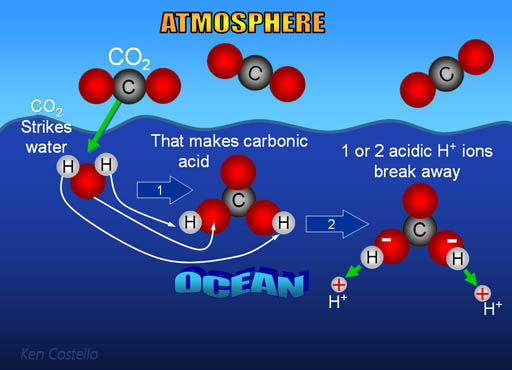
SOLVED: Carbon dioxide reacts with water in our blood to form carbonic acid. This reaction is critical in maintaining constant pH values Water Hydrogen ion HzCOz H+ HCO3 Carbonic Bicarbonate acid ion
Carbonic Acid Molecule. Formed when Carbon Dioxide is Dissolved in Water (carbonated Water). 3D Rendering Stock Illustration - Illustration of formula, atomic: 187047891
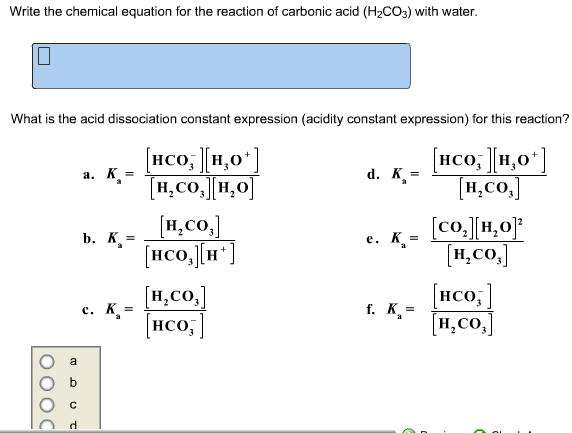
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora

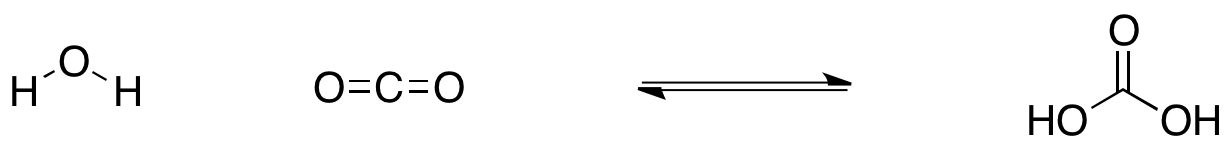
Carbonic acid can form water and carbon dioxide upon heating. how much carbon dioxide is formed from 6.20 g - Brainly.com
Carbonic acid (H2CO3) molecule . It is also solution of carbon dioxide in water (carbonated water). Structural chemical formula and molecule model. V Stock Vector Image & Art - Alamy