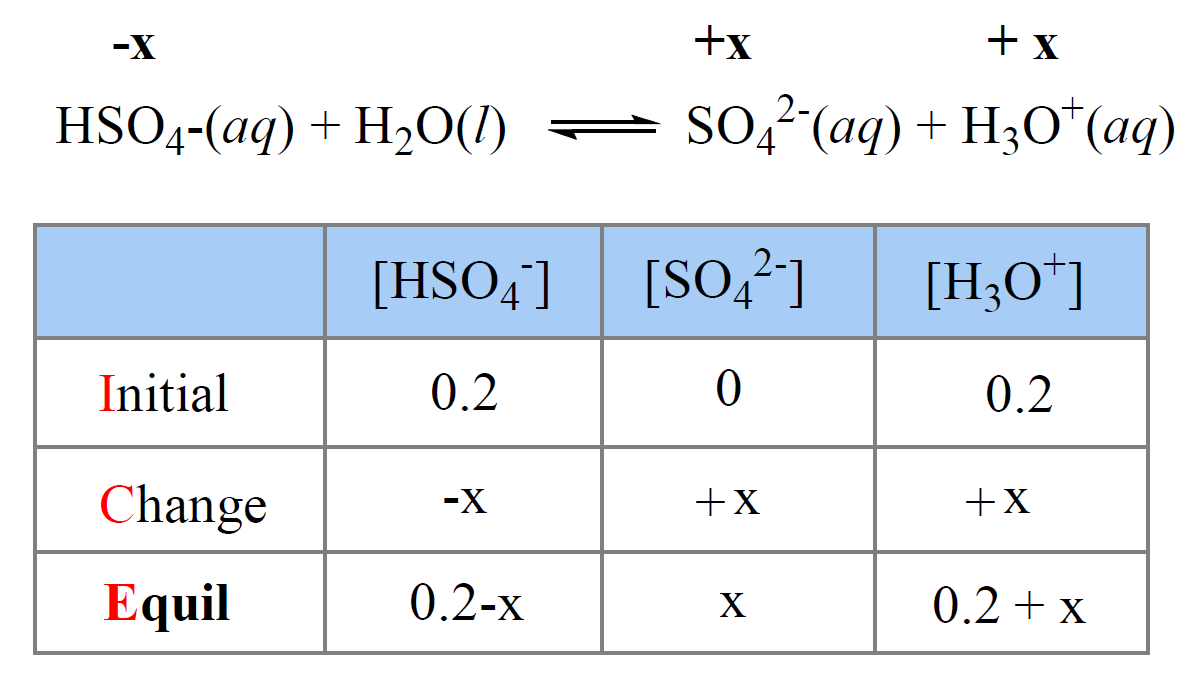
Using the ICE Chart to solve the concentrations for a weak acid or base. Different because earlier you were given concentrations and asked to solve for. - ppt download

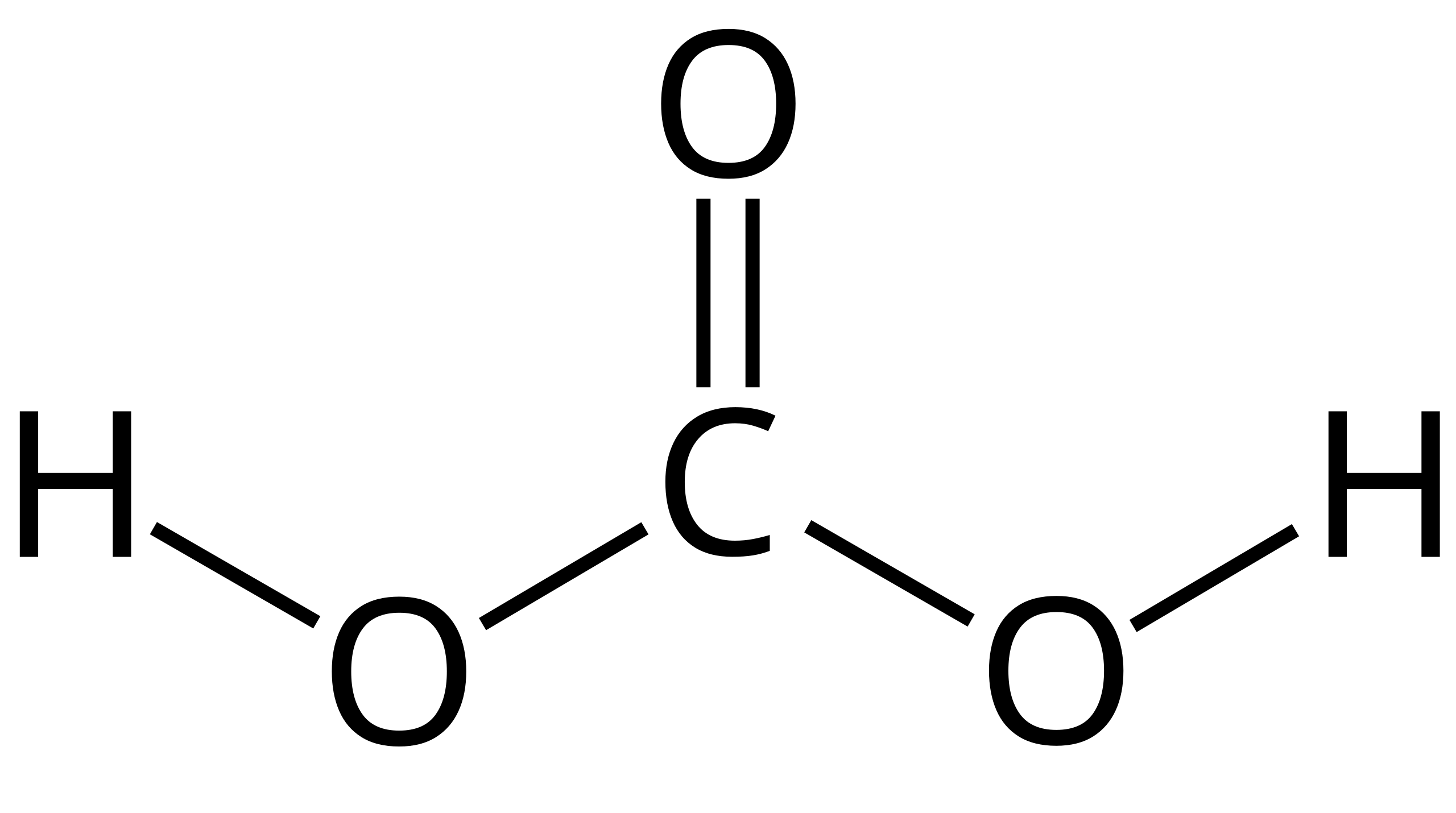
SOLVED: acid chemical formula Ka acetic butyric carbonic formic hypochlorous monohydrogen carbonate nitrous propionic HCzH3Oz HCAH-Oz HzCOs HCHO HCIO HCOz 1.8 x 10 15*10- 43x 10-7 1.8 x 10-4 3.0 x 10-8

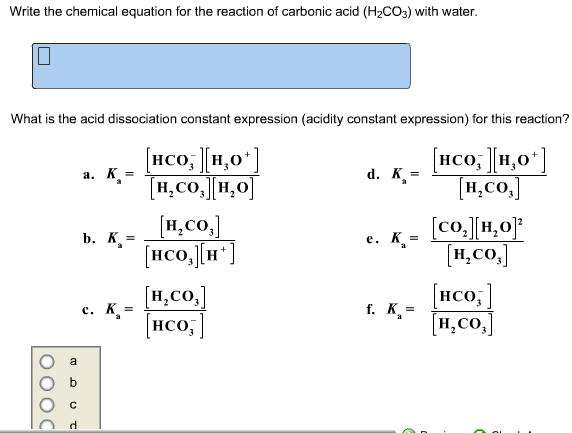
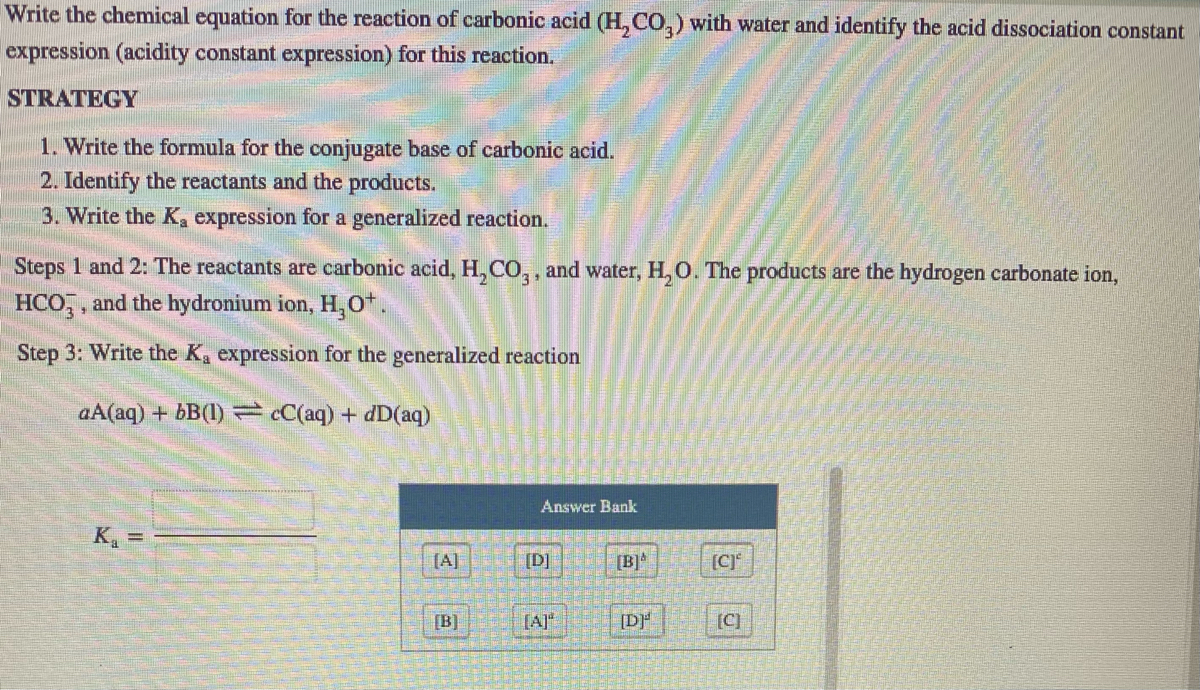
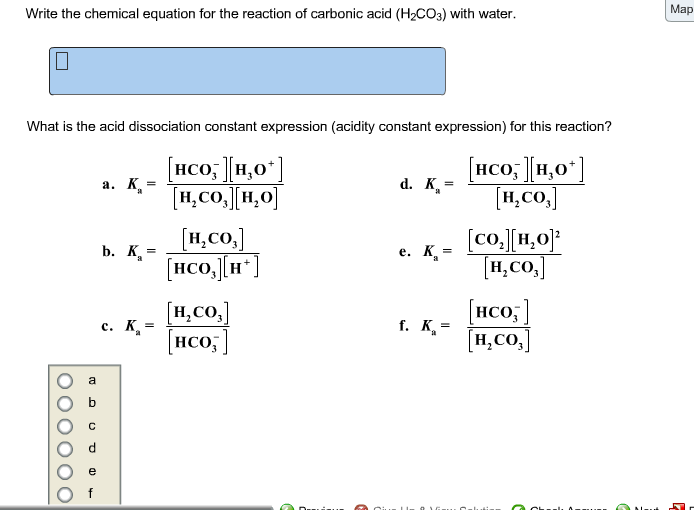
Question 26 of 32What is the equation for the acid dissociation constant, Ka, of carbonic acid? - Brainly.com
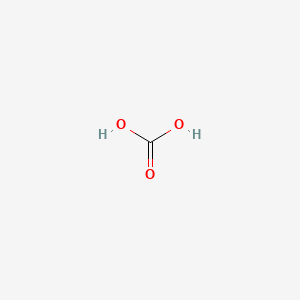
![SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H - SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H -](https://cdn.numerade.com/ask_images/4aa036e142eb4832ae078b434fceb3cf.jpg)
SOLVED: The equilibrium constant for the first step dissociation of Carbonic Acid could be written as Point) Note Chemical formula of Carbonic Acid is HzCO3 [H - 1+co; | [H-COs] Kal H -

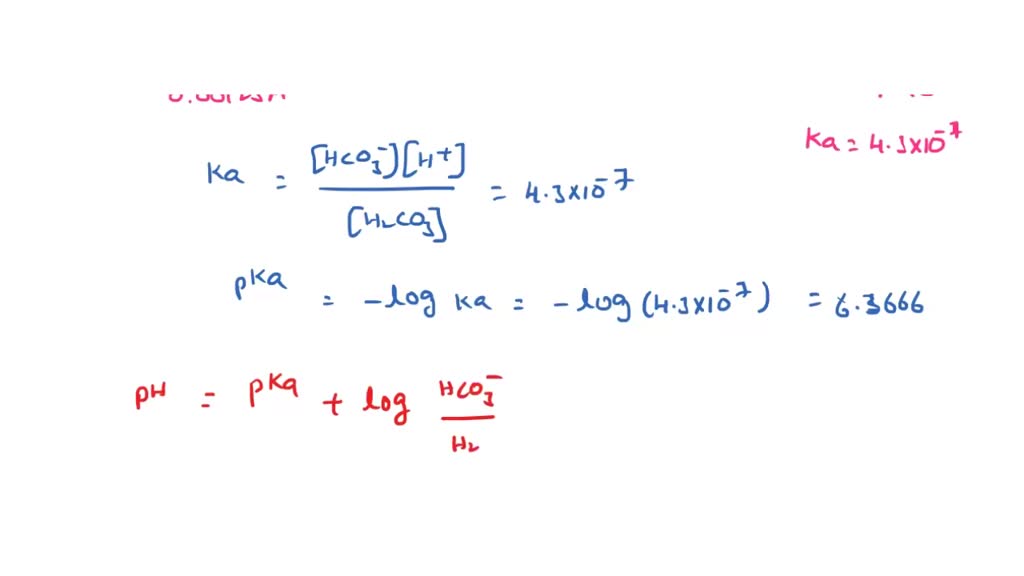
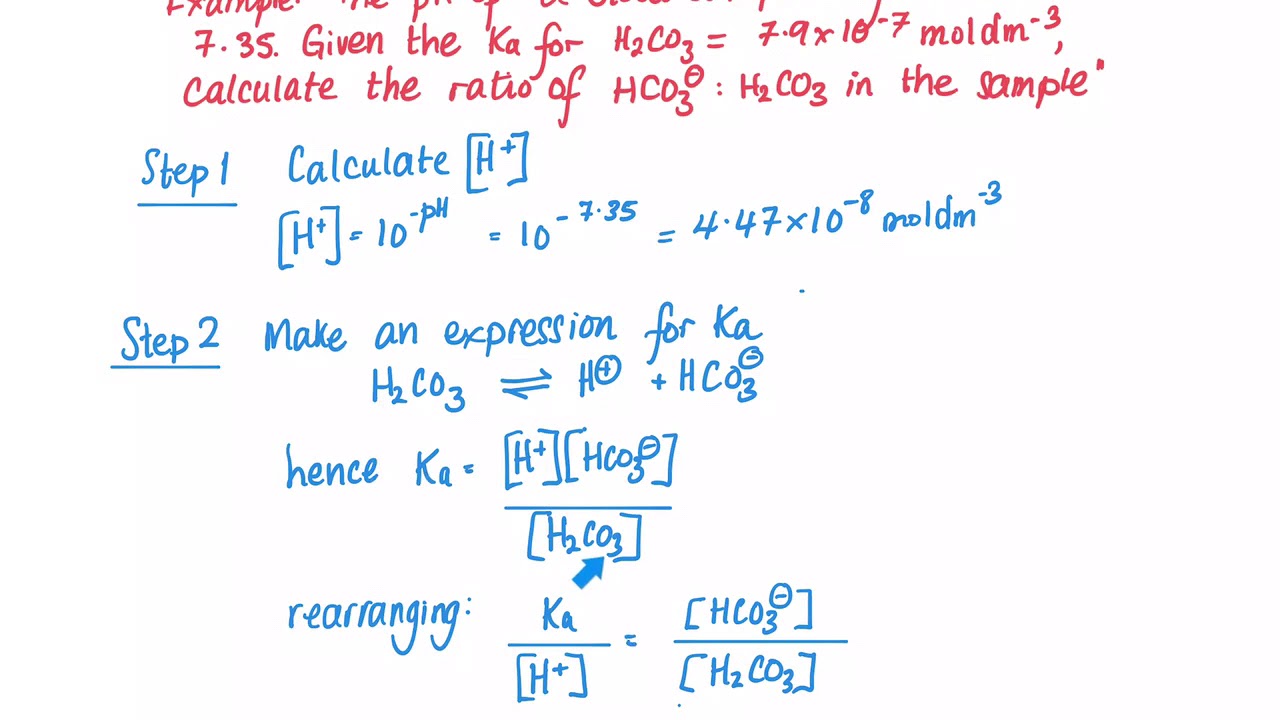
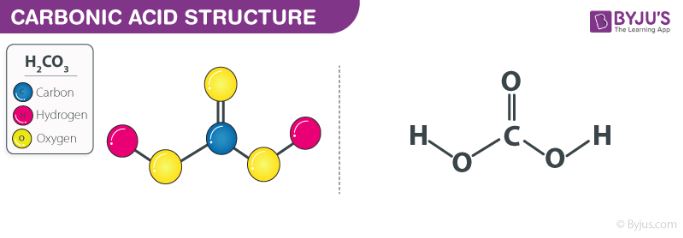
OneClass: Consider the acid dissociation behavior of carbonic acid, H2CO3. pKa 6.351 pka2 10.329 HO C...
![Carbonic acid (H2CO3) , a diprotic acid has Ka1 = 4.0 × 10^-7 and Ka2 = 7.0 × 10^-11 . What is the [CO3^2 - ] of a 0.025 M solution of carbonic acid? Carbonic acid (H2CO3) , a diprotic acid has Ka1 = 4.0 × 10^-7 and Ka2 = 7.0 × 10^-11 . What is the [CO3^2 - ] of a 0.025 M solution of carbonic acid?](https://haygot.s3.amazonaws.com/questions/1867619_141738_ans_a79741394e824d72b5d0da92daec007a.jpeg)
Carbonic acid (H2CO3) , a diprotic acid has Ka1 = 4.0 × 10^-7 and Ka2 = 7.0 × 10^-11 . What is the [CO3^2 - ] of a 0.025 M solution of carbonic acid?

THE CO 2 -H 2 O SYSTEM - I Carbonic acid is a weak acid of great importance in natural waters. The first step in its formation is the dissolution of CO. - ppt download