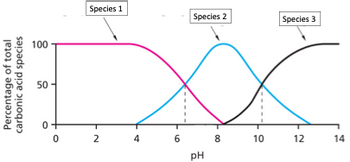
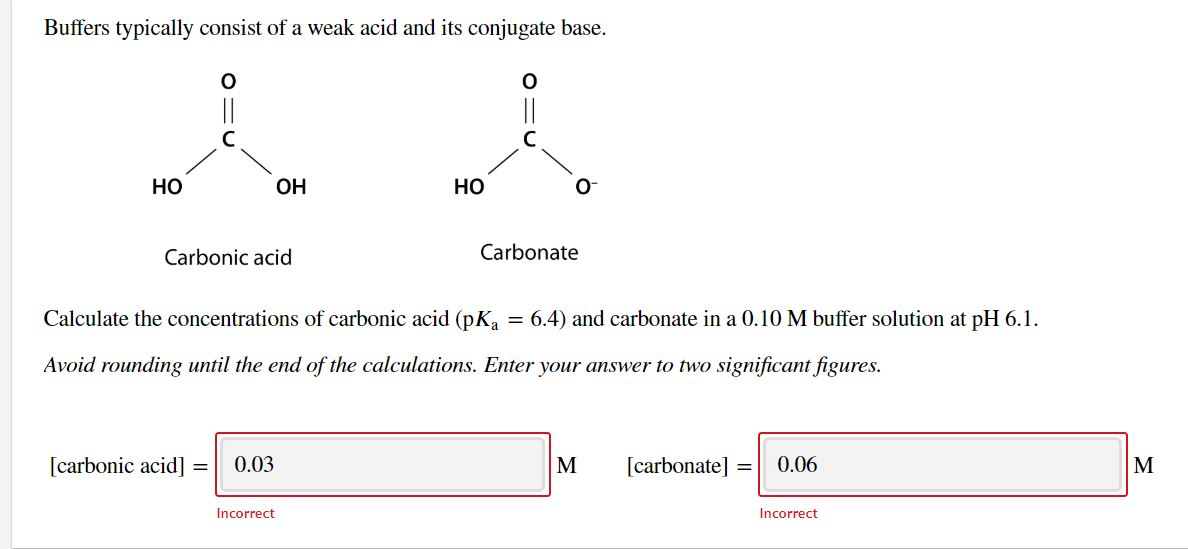
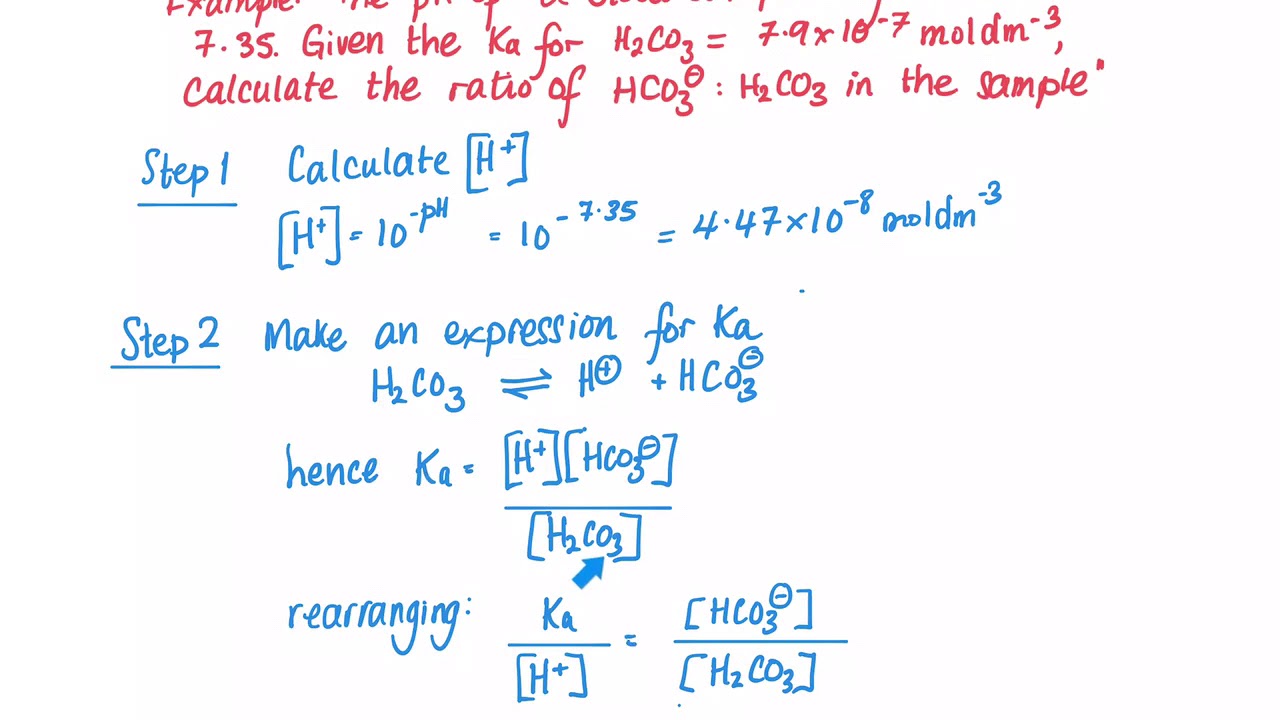
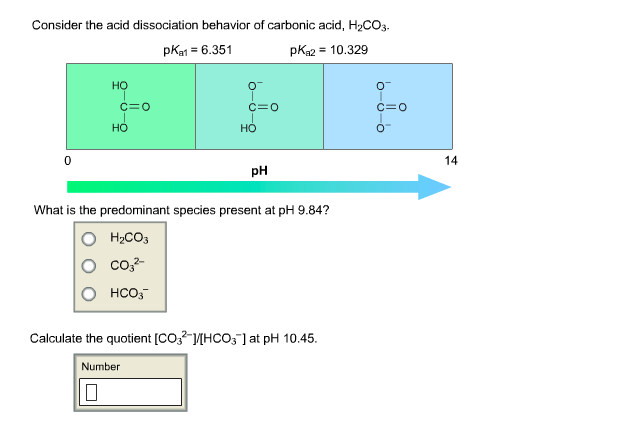
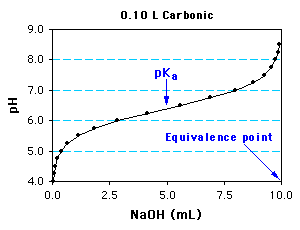
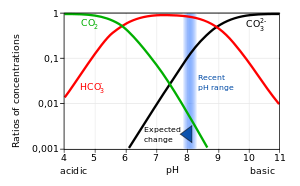
Color online) (A) Calculated speciation profile of carbonic acid as a... | Download Scientific Diagram
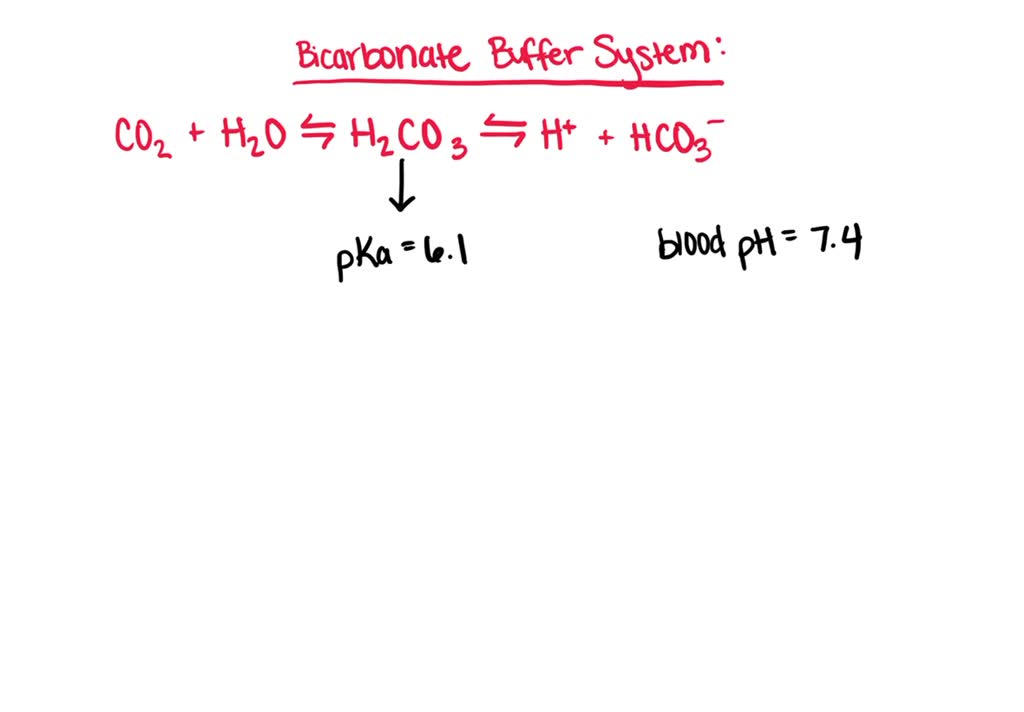
SOLVED: Carbonic acid has a pKa of 6.1 at physiological temperature. Is the carbonic acid/bicarbonate buffer system that maintains the pH of the blood at 7.4 better at neutralizing excess acid or
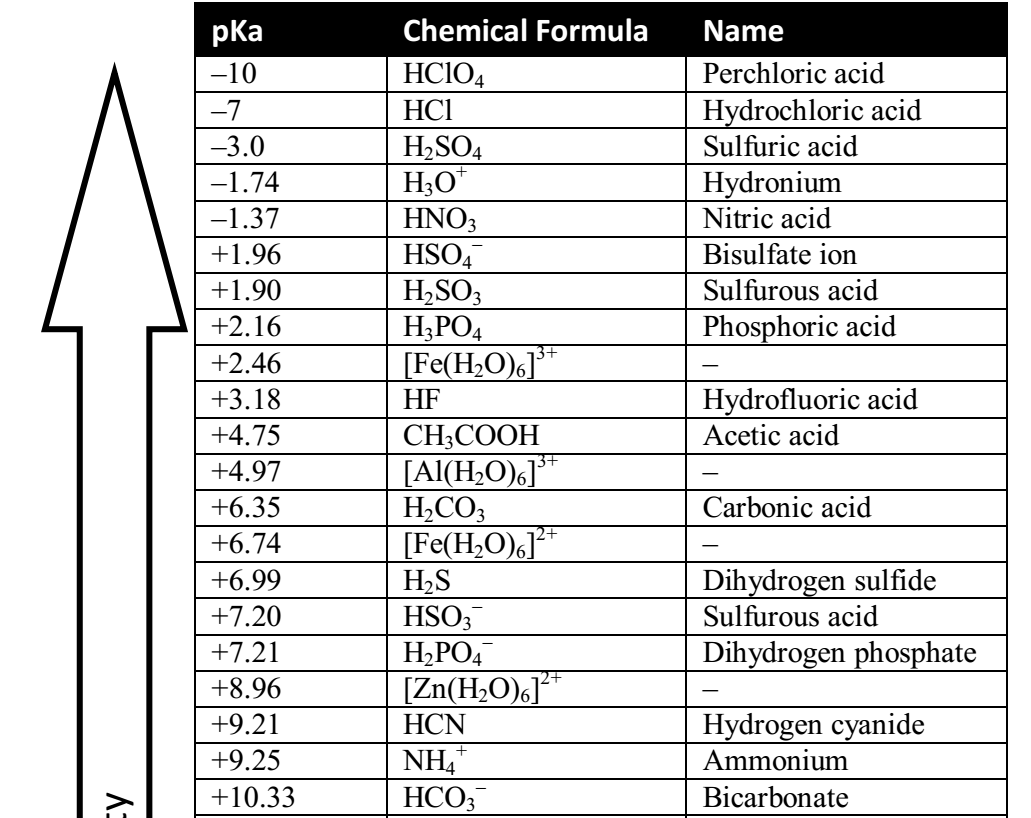
SOLVED: At a pH of 8.0, which of the following molecules is the dominant form of carbonic acid (pKa = 6.3, 10.3)? More than one is dominant CO3 2 - H2CO3 HCO3
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora

Biological buffering of blood There are three major contributors to regulating the pH of blood. Bicarbonate, phosphate and proteins Blood pH Must be Kept. - ppt download

physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange

The comparison of pKa determination between carbonic acid and formic acid and its application to prediction of the hydration numbers - ScienceDirect