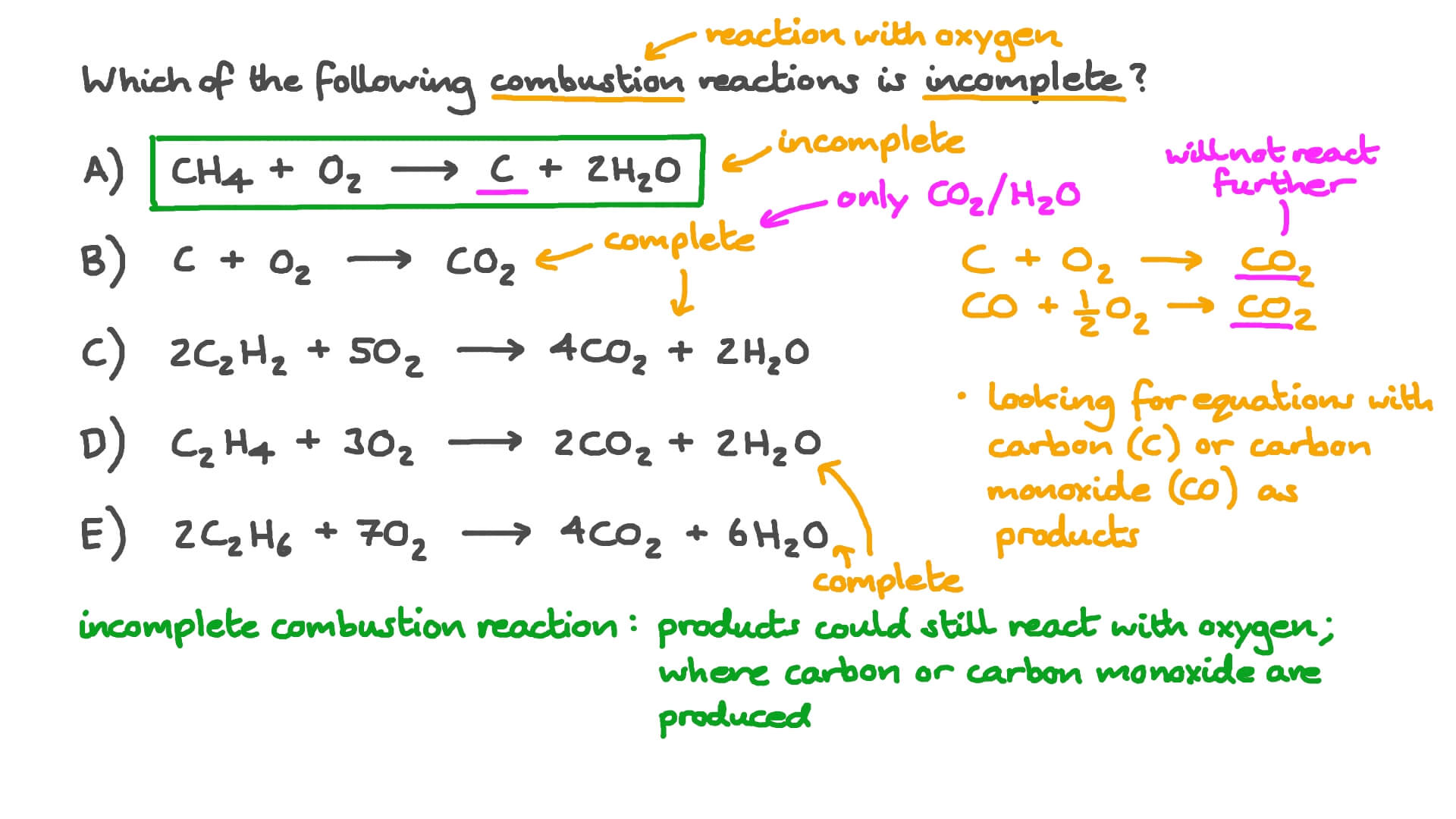
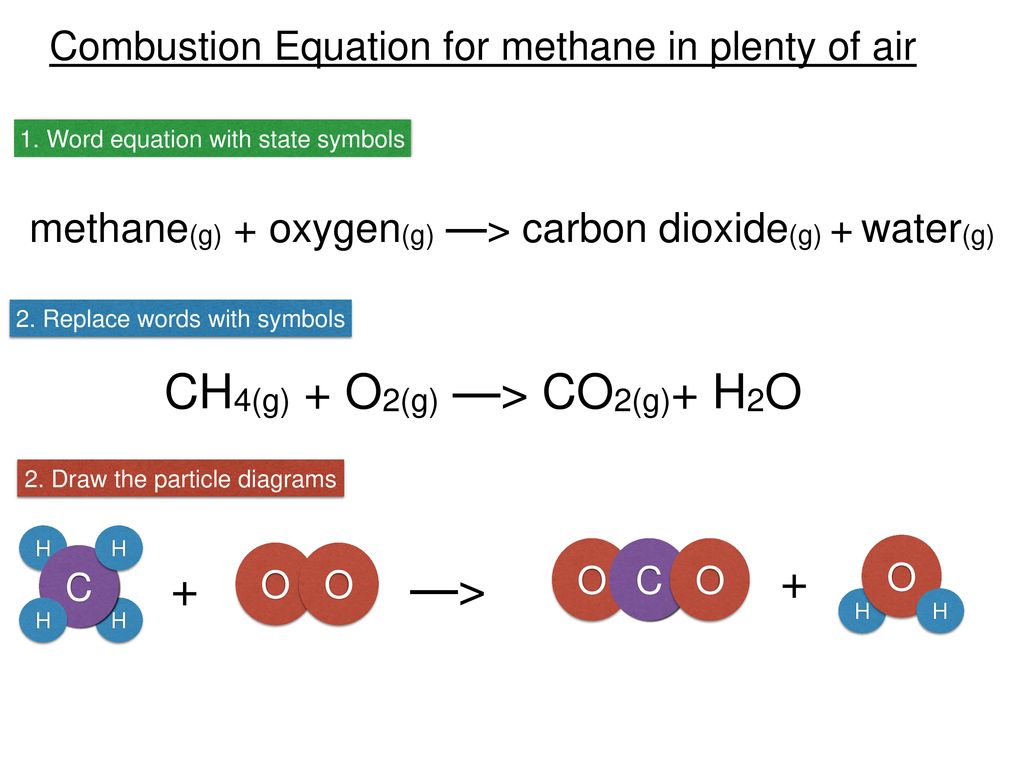
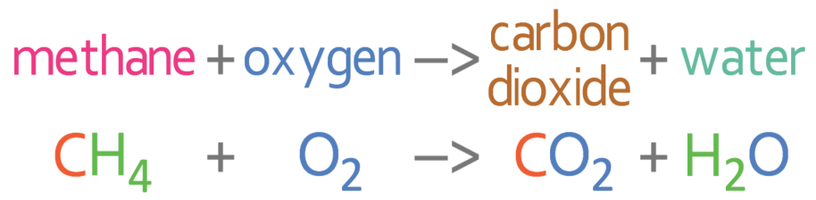The enthalpy of combustion of carbon and carbon monoxide are - 393.5 and - 283 kJ/mol respectively. The enthalpy of formation of carbon monoxide per mole is:
20. One mole of carbonundergoes incomplete combustion to produce carbon monoxide. Calculate(AH Δ ) for the formation of CO at 298 KGiven R 8314 JK 1 mol 1

The enthalpies of combustion of carbon and carbon monoxide are `-390 kJ mol^(-1)` and `-278 kJ mo - YouTube

How is the combustion of carbon a redox reaction? I get the oxidation part, but where is the reduction? - Quora
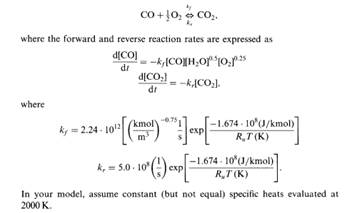
Solved) - Develop a model of the combustion of carbon monoxide with moist... - (3 Answers) | Transtutors
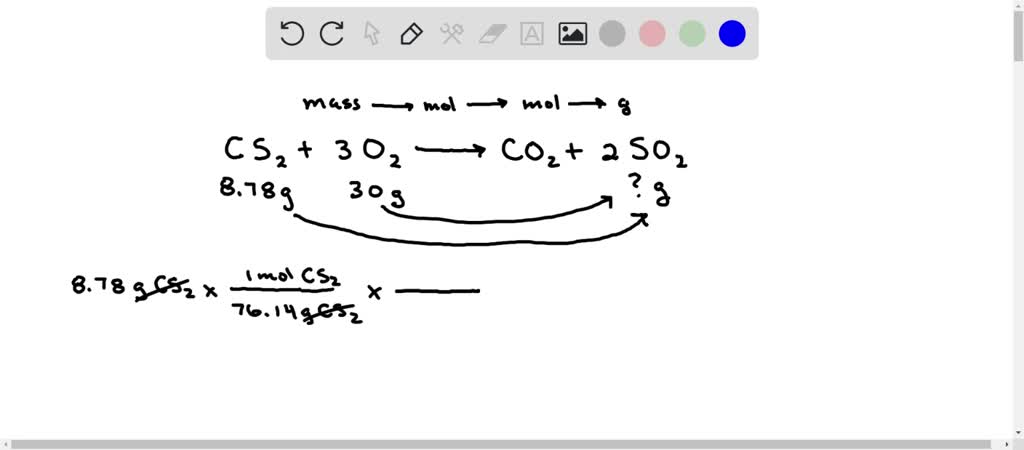
SOLVED: The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide according to the following UNBALANCED reaction: CS2 (g) + 3O2 (g) → CO2 (g) +