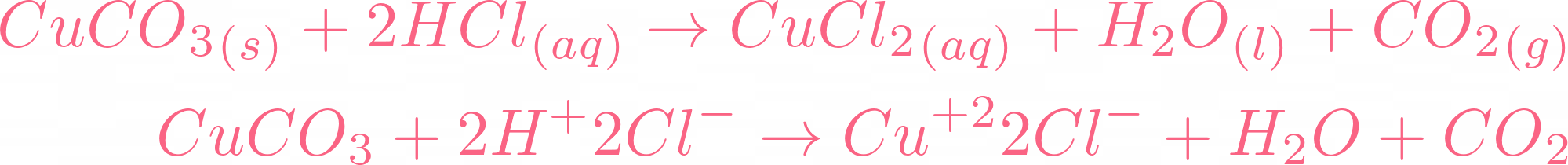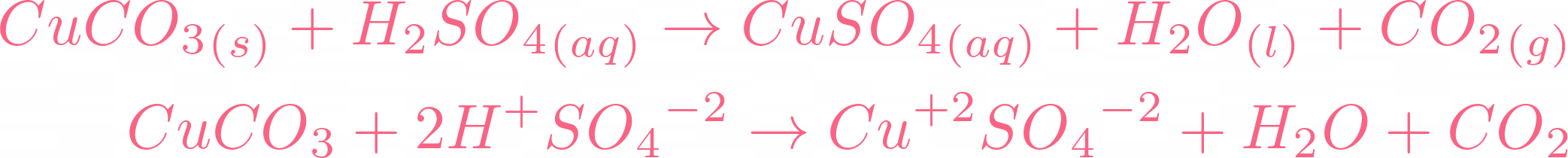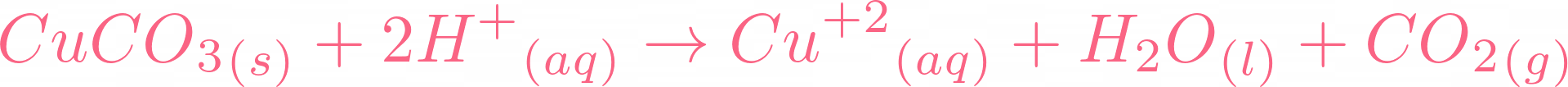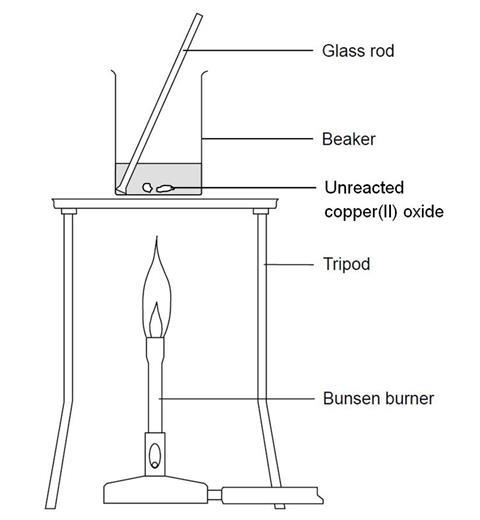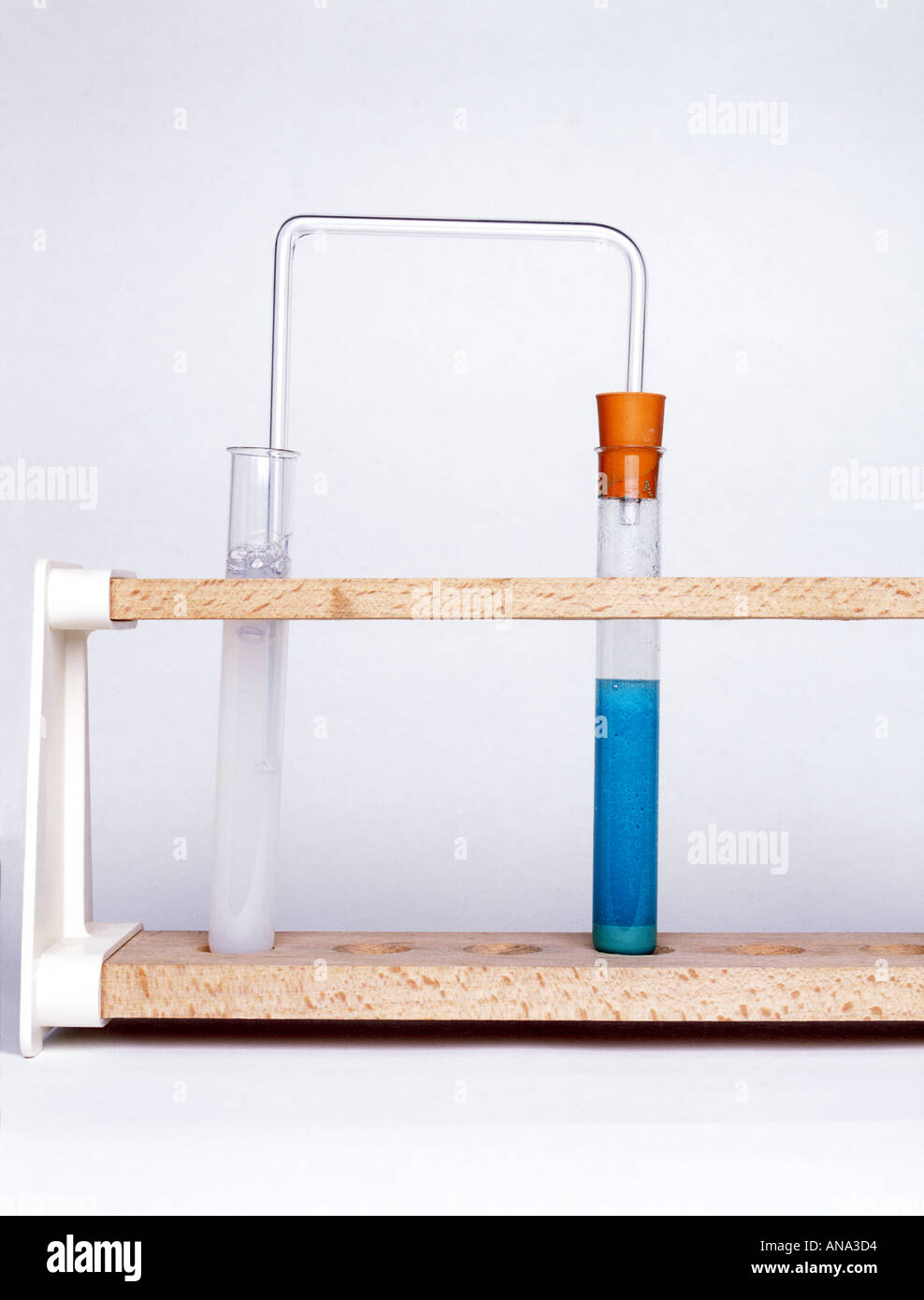
copper carbonate reacts with sulphuric acid to produce carbon dioxide which bubbles through limewater turning it milky Stock Photo - Alamy

Most carbonates are insoluble (can not be dissolved in water) except those containing sodium or potassium ions. - ppt download
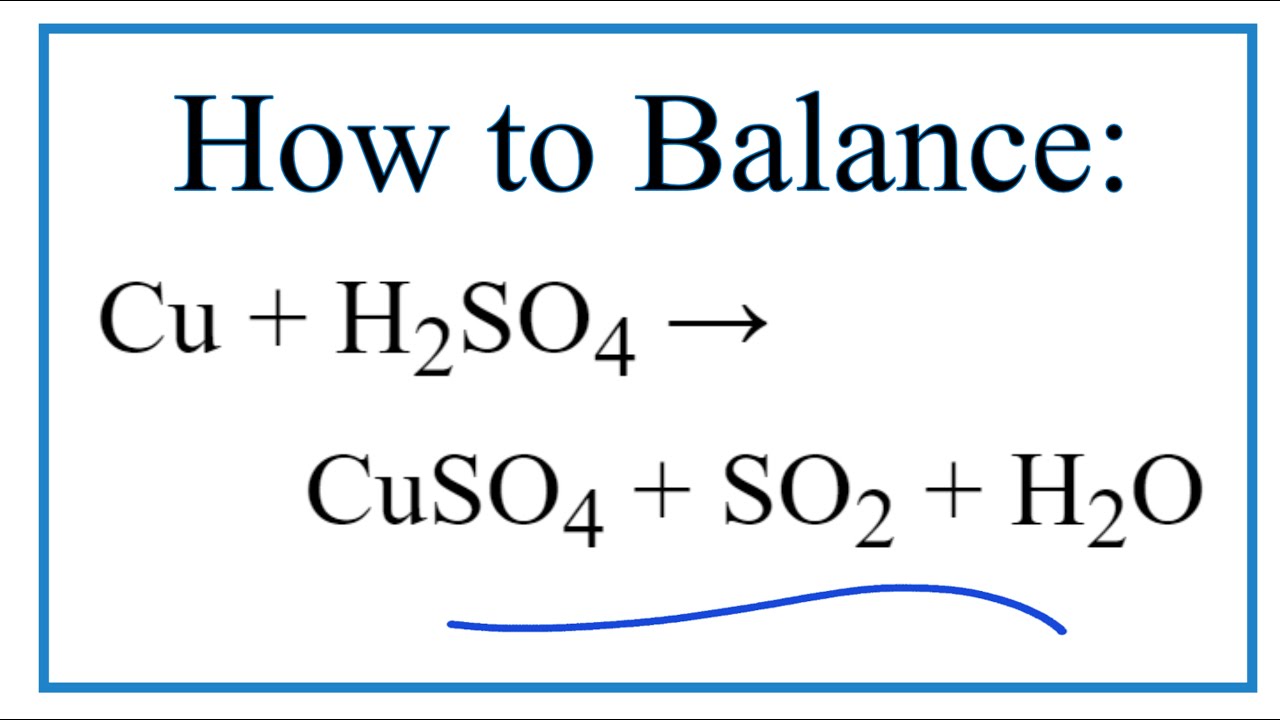
i) Lead sulphate from lead carbonate (ii) Sodium sulphate using dilute sulphuric acid. (iii) Copper chloride using copper carbonate. - Sarthaks eConnect | Largest Online Education Community
![Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition](http://img.youtube.com/vi/9tJGRn8Su8s/0.jpg)
Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition

Write word equations and then balanced equations for the reaction taking place when:(a) Dilute sulphuric acid reacts with zinc granules.(b) Dilute hydrochloric acid reacts with magnesium ribbon.(c) Dilute sulphuric acid reacts with
![Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/05/mq2-3.jpg?w=640)
Chemical Reactions to make copper(II) sulfate| Chemistry Basics [Online Video] – O Level Secondary Chemistry Tuition

Q3 Give reasons why the following are considered as chemical changes 1 Copper Carbonate on heat give...